Molecular Properties¶
Info
Many properties of a compound are directly related to its 3D structure. Examples are given in different fields including biological, physical and chemical properties
Number of Pages: 17 (±0 hours read)
Last Modified: January 2004
Prerequisites: None
Introduction¶
Properties of a Molecule¶
Molecules are defined by 2D chemical formulas but the information they carry is not sufficient for understanding the physical, chemical, or biological properties of the compound. Molecules are 3D entities and only their 3D geometries provide relevant information for understanding such properties.
Average of a Conformational-Dependent Property¶
Molecular properties are directly related to the 3D structure of the molecule. When a molecule exists as a mixture of conformers, then any property that this molecule exhibits is an average of that property over all of the conformations it visits, weighted by the conformers populations.
Importance of the 3D Molecular Geometries¶
The following pages illustrate the relationships between the 3D molecular geometry of molecules with various properties.
Biological Properties¶
Biological Properties of Proteins¶
The function of a protein is controlled by its three-dimensional structure. Enzymes and receptors recognize substrates on the basis of their 3D molecular geometries. Drugs also are designed on the basis of their precise 3D properties.
Biological Properties of Chiral Analgesics¶
Even very similar compounds such as isomers or stereoisomers may have very different biological properties. Ibuprofen exists as a racemic mixture, only the S enantiomer has analgesic properties. The biological activity/inactivity is directly related to the molecular geometries of the two enantiomers.
articles
Stereochemistry in the Analysis of Drug Action Ariens EJ Med. Res. Rev. 7 1987
New Resolutions in Drug Design Carey J Chemistry in Britain Dec 1993
Chiral Drugs Stinson SC Chem. Eng. News 72(38) 1994
When Drug Molecules Look in the Mirror Thall E J. Chem. Ed 73 1996
Pharmaceutical development and specification of stereoisomers Williams RC, Riley CM, Sigvardson KW, Fortunak J, Ma P, Nicolas EC, Unger SE, Krahn DF and Bremner SL J. Pharm. Biomed. Anal 17(6-7) 1998
Physical Properties¶
Physical Properties¶
The boiling point, the refractive index or the magnetic susceptibility are physical properties that can be derived from the molecular geometries of the molecules. For example the boiling point of iso-butane (-12o C) is lower than that of n-butane (-1o C). This is due to the difference in the molecular structures of the two isomers. Isobutane is almost spherical and has less intermolecular forces than n-butane which has an extended geometry.
articles
Molecular Structure-Property Relationships for Alkenes Nelson SD and Seybold PG J. Mol. Graph. Model. 20(1) 2001
The Relationship Between Redox Potentials and Torsional Angles in 4,4'-Dimethyl N,N'-Alkylidene 2,2'-Bipyridinium salts Wang Y and Zhao W Molecules 5 2000
Calculation of Other Physical Properties¶
Theoretical treatments facilitate the calculation of a number of molecular properties and require as input the molecular geometry of the molecule concerned. The properties that can be calculated are electronic properties (ionization potentials, atomic charges, electrostatic potentials, molecular orbitals) or thermodynamic properties (activation energies, enthalpies, entropy, free energies, heat of formation). The picture shows the partial atomic charges for this representative molecule.
articles
Atomic Charges Derived from Electrostatic Potentials: A Detailed Study Chirlian LE and Francl MM J. Comput. Chem. 8 1987
Solubilit{eacute} des Mol{eacute}cules D{eacute}termin{eacute}es {agrave} Partir de leurs Caract{eacute}ristiques G{eacute}om{eacute}triques et Electroniques: Cas des Hydrocarbures dans l'Eau Cohen NC and Regnier G Bull. Soc. Chim. France
1976
Representation of the Molecular Electrostatic Potential by a Net Atomic Charge Model Cox S and Williams D J. Comput. Chem. 2 1981
Iterative Partial Equalization of Orbital Electronegativity-A Rapid Access to Atomic Charges Gasteiger J and Marsili M Tetrahedron 36 1980
Classical Electrostatics in Biology and Chemistry Honig B and Nicholls A Science 268(5214) 1995
Theoretical Characterization of the Electronic Properties of Extended Thienylenevinylene Oligomers Krzeminski C, Delerue C, Allan G, Haguet V and Stievenard D J. Chem. Phys. 111(14) 1999
Electronegativity Equalization Method for the Calculation of Atomic Charges in Molecules Mortier WJ, Ghosh SK and Shankar S J. Am. Chem. Soc. 108 1986
Multipole Expansion Techniques for the Calculation and Characterization of Molecular Electrostatic Potentials Rabinowitz JR and Little SB Int. J. Quantum Chem. Quantum. Biol. Symp. 13 1986
A Finite Expansion Method for the Calculation and Interpretation of Molecular Electrostatic Potentials Rabinowitz JR, Namboodiri K and Weinstein H Int. J. Quantum. Chem. 29 1986
The Electrostatic Molecular Potential as a Tool for the Interpretation of Molecular Properties Scrocco E and Tomasi J Topics Curr. Chem. 42 1973
Chemical Properties¶
Chemical Properties¶
A chemical compound has physical and chemical properties that are determined by its precise chemical constitution and architecture. Chemical reactions occur in three-dimensions. The way a chemical reaction develops can be explained by analyzing the reactants and their interactions in three dimensions. This is so important that chemists try to incorporate this information into their two dimensional diagrams using some additional conventions.
articles
The Geometry of N-hydroxymethyl Compounds. Part 3: Geometries of N-(hydroxymethyl)-amides Related to Reactivities Mallawaarachchi W, Simmonds RJ and Parry DE Anti. Can. Drug. Des. 4 1989
Enolization of Keto-3 Steroids¶
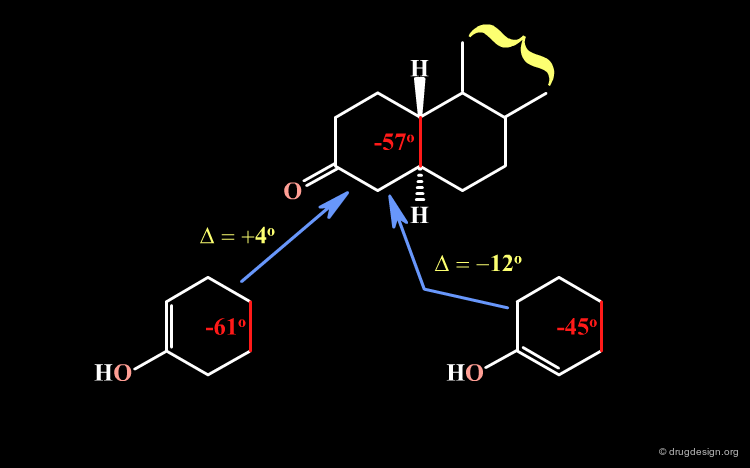
The enolization of keto-3 steroids occurs as a mixture of the two enolic forms. There is a greater proportion of the enol with the double bond in the Δ2 position than the one in the Δ3. The explanation is given on the following pages.
articles
249. Steroids. Part CLXXI. Factors Controlling the Direction of Enol Acetylation of 3-oxo-steroids Berkoz B, Chavez EP and Djerassi C J. Chem. Soc.
1962
The Chemistry of Carbanions. IX. The Potassium and Lithium Enolates Derived from Cyclic Ketones House HO and Trost BM J. Org. Chem. 30 1965
Relative Stability of Isomers¶
A qualitative analysis allows us to explain this preference. At the fusion with ring B the torsion angle of ring A has a value of about -57o; it should become -61o (left bottom) or -45o (right bottom) after the reaction, which correspond to a variation (in absolute values) of 4o and 12o, respectively. Assuming that the 4o variation will be preferred, this qualitative analysis explains why there are greater proportions of the Δ2 enol.
articles
249. Steroids. Part CLXXI. Factors Controlling the Direction of Enol Acetylation of 3-oxo-steroids Berkoz B, Chavez EP and Djerassi C J. Chem. Soc.
1962
The Chemistry of Carbanions. IX. The Potassium and Lithium Enolates Derived from Cyclic Ketones House HO and Trost BM J. Org. Chem. 30 1965
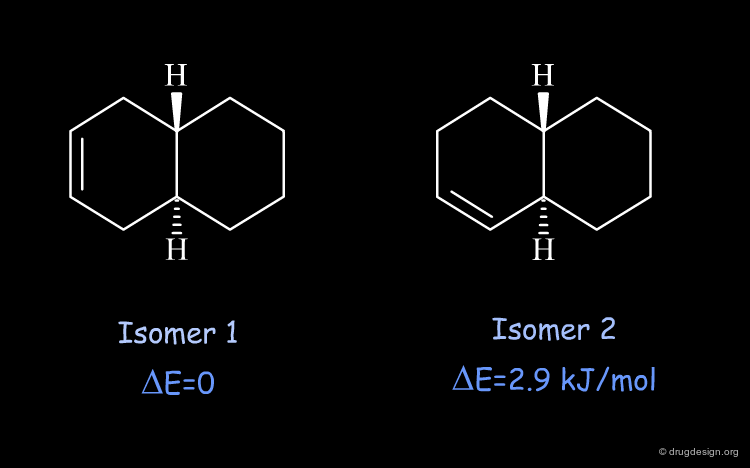
Relative Stability of the two Isomers of Trans-Octalin¶
The relative stability of the two isomers of trans-octalin can be calculated based on the 3D geometries of the specified compounds. There is a difference of energy of 2.9 kJ/mol in favor of isomer 1, which is the more stable because it has less strain in the bicyclic moiety.
Geometrical Preference Explains Enolization¶
The geometrical preference for the Δ2 isomer explains why the enolization of β-Decalone produces a Δ2/Δ3 enol ratio of 72%/28%.
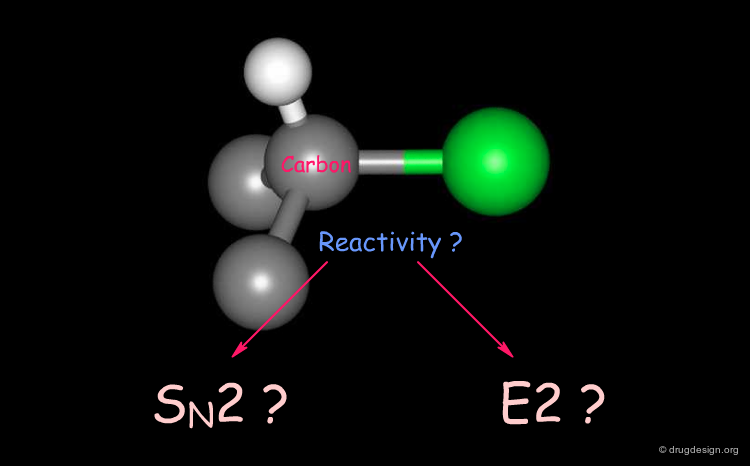
Reactivity of Alkyl Halides¶
Alkyl halides react in a totally different manner depending on the geometrical accessibility of the carbon connected to the halogen atom.
articles
Reaction Kinetics and the Walden Inversion. Part VI. Relation of Steric Orientation on Mechanism in Substitutions Involving Halogen Atoms and Simple or Substituted Hydroxyl Groups Cowdrey WA, Hughes ED, Ingold CK, Masterman S and Scott AD J. Chem. Soc.
1937
A Steric Model for the Prediction of Stereoselectivity at the Carbonyl Carbons in Cyclic Compounds Lagerstedt IC and Olsson T J. Chem. Inf. Comput. Sci. 33 1993
Computing Reaction Pathways on Molecular Potential Energy Surface McKee ML and Page M Reviews in Computational Chemistry 4 1993
Gas-Phase Nucleophilic Displacement Reactions Riveros JM, Jose SM and Takashima K Adv. Phys. Org. Chem. 21 1985
Distinguishing Between Concerted and Nonconcerted Elimination Saunders WH Jr Accounts of Chemical Research 9 1976
Bimolecular Elimination Reaction: Stereochemistry and the Scope of Conformational Analysis Sicher J Pure. Appl. Chem. 25 1971
The syn and anti Steric Course in Bimolecular Olefin-Forming Elimination Sicher J Angew. Chem. Internal. Edit. 11 1972
Gas-Phase Acid-Induced Nucleophilic Displacement Reaction. Stereochemistry of Inter- and Intramolecular Substitutions at Saturated Carbon Speranza M and Angelini G J. Am. Chem. Soc. 102(9) 1980
SN2 Mechanism¶
The nucleophilic attack of R-1-Bromo-1-deuterioethane by an ethoxy ion occurs by a SN2 mechanism. The carbon atom is accessible for a nucleophilic attack and in the final product its stereochemistry has been inverted.
E2 Elimination Mechanism¶
The same reaction with tert-butylbromide gives a different reaction because for steric reasons the central carbon atom is not accessible. What is observed here is not an SN2 reaction as in the previous case but an elimination of HBr according to an E2 elimination mechanism.
Molecular Geometries and Chemical Properties¶
Theoretical treatments facilitate the calculation of a number of molecular chemical properties. These properties are 3D dependent and include physico-chemical indexes (solubility, lipophilicity), interconversion energies (rotational barriers, ring inversions) and chemical reactivity (electronic indexes, lipophilicity, reaction paths, relative stability of isomers).
articles
Application of Molecular Mechanics Calculation to Organic Chemistry Osawa E and Musso H Topics in Stereochemistry 13 1982
Molecular Electrostatic Potentials and Chemical Reactivity Polizer P and Murray JS Reviews in Computational Chemistry 2 1991
The Use of the Electrostatic Potential at the Molecular Surface to Interpret and Predict Nucleophilic Processes Sjoberg P and Politzer P J. Phys. Chem. 94 1990
Computational Molecular Dynamics of Chemical Reaction in Solution Whitnell RM and Wilson KR Reviews in Computational Chemistry 4 1993
book
Warshel A Comptuter Modeling of Chemical Reactions in Enzymes and Solutions Wiley 1991
Many Properties¶
Many Properties of a Molecule¶
A molecule has many properties that are directly related to its geometry. They can be obtained either experimentally or through theoretical calculations.
articles
Computational Approaches to Lipophilicity: Methods and Applications Carrupt PA, Testa B and Gaillard P Reviews in Computational Chemistry 11 1997
Theoretical Methods fro Computing Enthalpies of Formation of Gaseous Compounds Curtiss LA, Redfern PC and Frurip DJ Reviews in Computational Chemistry 15 2000
3-D Molecular Lipophilicity Potential Profiles, A new Tool in Molecular Modeling Furet P, Sele A and Cohen NC Journal of Molecular Graphics 6 1988
A Fast Empirical Method for the Calculation of Molecular Polarizability Glen RC Journal of Computer-Aided Molecular Design 8 1994
The Coding of the Three Dimensional Structure of Molecules by Molecular Transforms and It's Application to Structure-Spectra Correlations and Studies of Biological Activity Schuur JH, Selzer P and Gasteiger, J J. Chem. Inf. Comput. Sci. 36 1996
Toward a Better Understanding of Covalent Bonds: The Molecular Mechanics Calculation of C-H Bond Lengths and Stretching Frequencies Thomas HD, Chen K and Allinger NL J. Am. Chem. Soc. 116 1994
Copyright © 2024 drugdesign.org