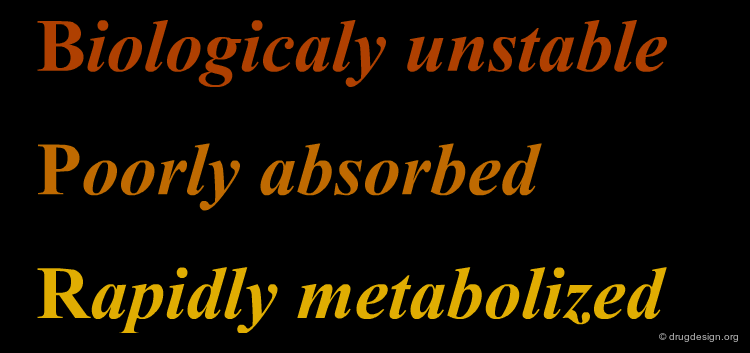Peptidomimetics: Principles and Methods¶
Info
Introduction of the concept of peptidomimicry, whose aim is to mimic a reference peptide compound by a non-peptidic molecule.
Number of Pages: 33 (±0 hours read)
Last Modified: January 2009
Prerequisites: None
Introduction¶
Key Peptides in Drug Discovery¶
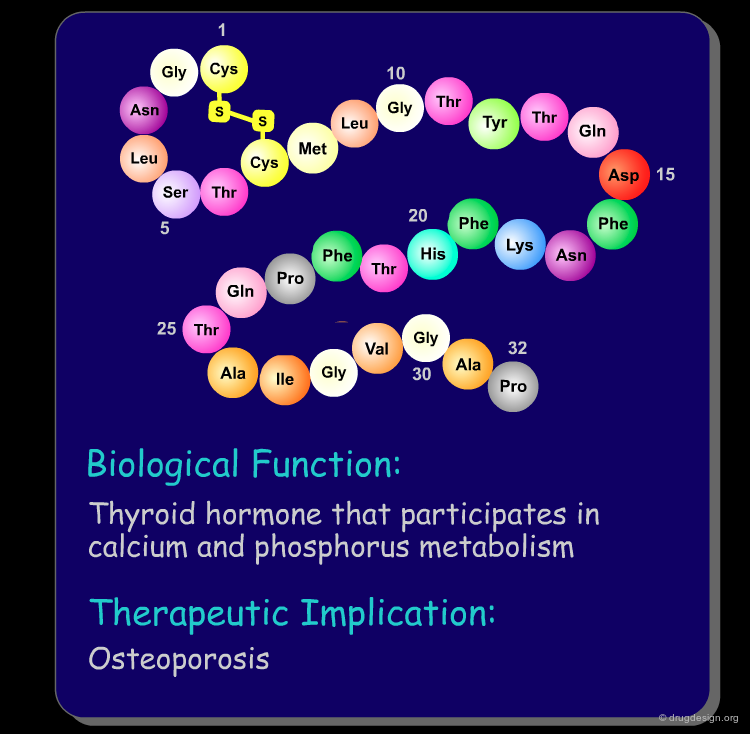
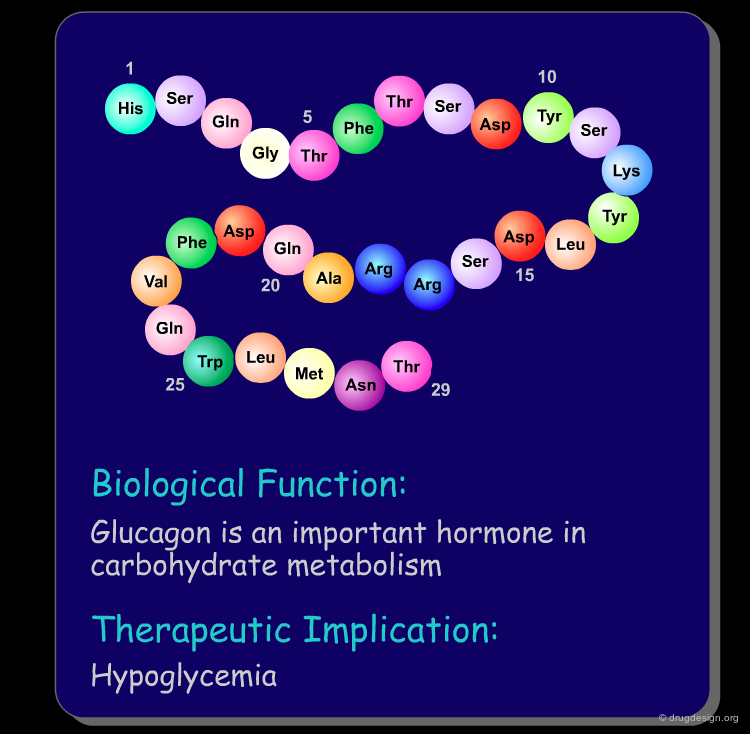
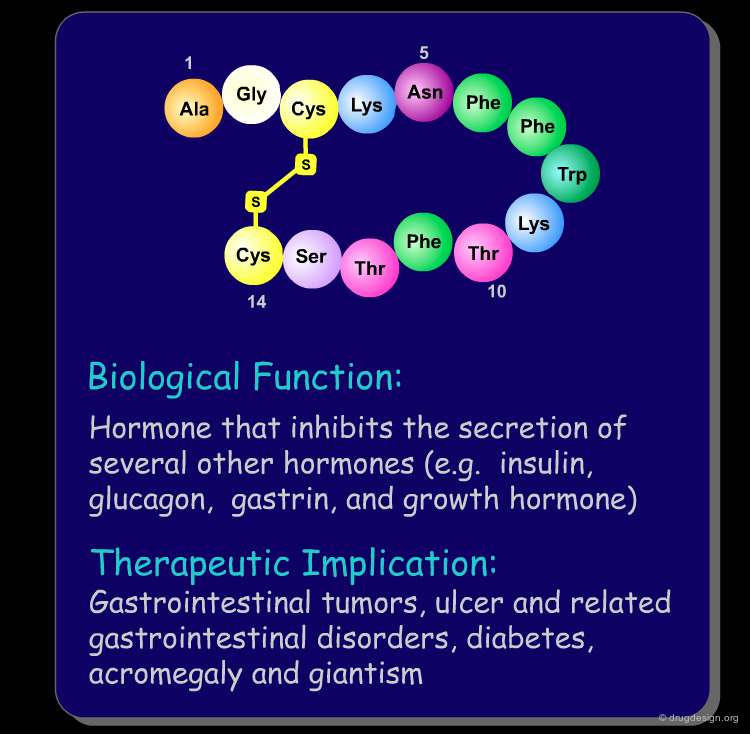
Peptide molecules are an essential part of the biological processes. They act as substrates in many enzymatic reactions and can regulate the activation and inhibition of many biological pathways. As such, peptides are attractive targets as therapeutic drugs and drug-leads (see examples below). Furthermore, if one starts with a peptidic substance, it is relatively easy to find new peptidic analogs that are also biologically active.
Definition of Peptidomimetics¶
Drug development sometimes requires the imitation of a reference peptide molecule. "Peptido-mimicry" or peptidomimetics is defined as the process of mimicking the biological properties of a peptidic substance.
Problems with Peptide Molecules¶
However, peptide compounds cannot be developed as drugs. Despite the excellent biological tests demonstrated in the laboratory, peptide molecules yield very poor results when the molecules are entered in clinical trials. The reason is that poor pharmacokinetics (including rapid proteolysis, metabolism, poor transport properties and rapid excretion) hinder the development of peptide and peptide-like molecules. In summary, peptidic molecules are:
The Aim of Peptidomimetics¶
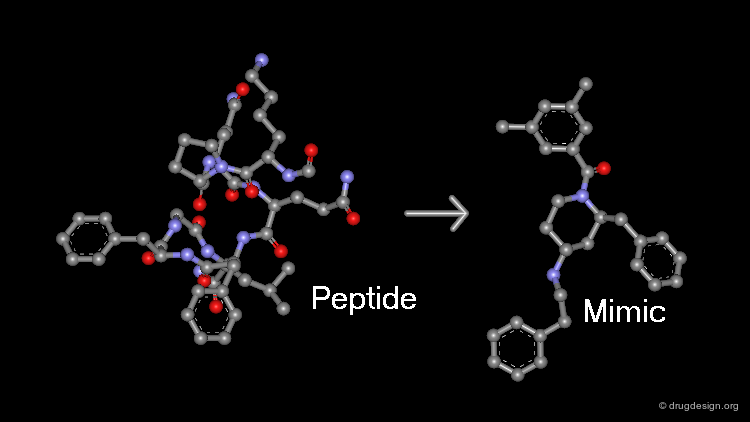
The aim of peptidomimetics is to find a non-peptidic molecule that mimics the action of a reference peptide ligand. A non-peptidic structure has also the advantage of providing the structural diversity necessary to allow optimization of specificity, oral bioavailability and pharmacokinetic properties. Also, in identifying an alternate series with different pharmacological properties, peptido-mimicry provides a powerful tool for creating proprietary molecules with a strong patent position.
Typical Peptidomimicry Projects¶
The following steps have been taken in many typical peptidomimicry projects: knowing the structure of a peptide molecule (a substrate or a hit from a phage-display library), a systematic synthetic chemistry program permitted the discovery of active small peptide analogs. Active peptide molecules can be easily discovered, however the question is how to make out of it a useful drug?
Two Alternative Routes¶
From Peptides to Non-peptidic Molecules¶
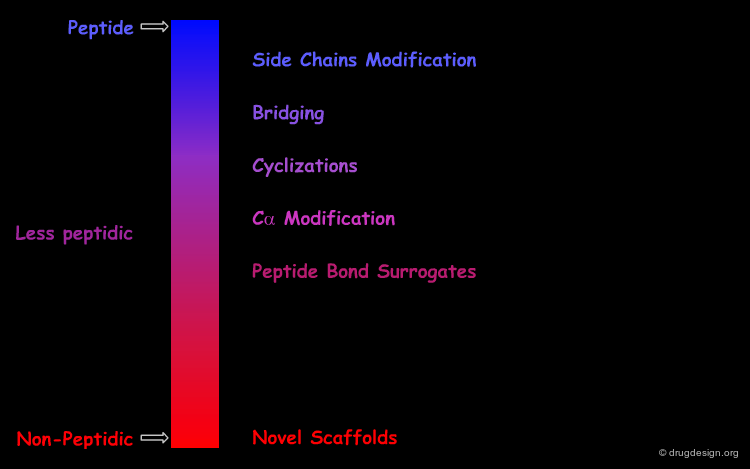
The entire spectrum of molecules is schematically represented in the following diagram. The initial peptide reference structures are on the left side while the ideal non-peptidic structures are located on the right side of the spectrum. The greater the distance, the less peptidic the molecule is. There are two routes for such transformations, which will be described in the next pages.
First Route: Successive Modifications of Peptide¶
Peptide-Like Examples¶
The first approach consists of successive modifications of the initial peptide, that will progressively reduce its peptide character in order to get a compound that is far enough from the starting reference.
Depeptidization¶
The successive modifications of the structure of a reference peptide is a complicated operation. It is sometimes called "depeptidization" and is relatively subjective. This approach requires a continuous effort of chemical syntheses.
Range of Structural Modifications¶
From a structural point of view, a peptide mimic can be designed by approaches ranging from relatively conservative replacements to more drastic modifications. Examples of selected approaches are demonstrated in the following pages. Each of these approaches can be the end-point of a peptidomimetic project or just one step in a sequence of modifications aiming at reducing the peptidic character.
articles
Converting a Peptide into a Drug: Strategies to Improve Stability and Bioavailability Adessi C and Soto C Curr. Med. Chem. 9 2002
Peptidomimetic-Tailored Enzyme Inhibitors Gante J Angew. Chem. Int. Ed. 33 1994
Peptidomimetics for Receptor Ligands Discovery, Development and Medical Prespectives Giannis A and Kotler T Angew. Chem. Int. Ed. 32 1993
Peptidomimetic Design. Ripka AS and Rich DH Curr. Opin. Chem. Biol. 2 1998
Peptidomimetics Derived from Natural Products. Wiley RA and Rich DH. Med. Res. Rev. 13 1993
Side Chain Mimicry¶
A relatively simple approach is to modify the side chains of various amino acids. The residues of interest are replaced by natural or non-natural amino acids with chemically and structurally similar side chains. The following are examples of some possible replacements.
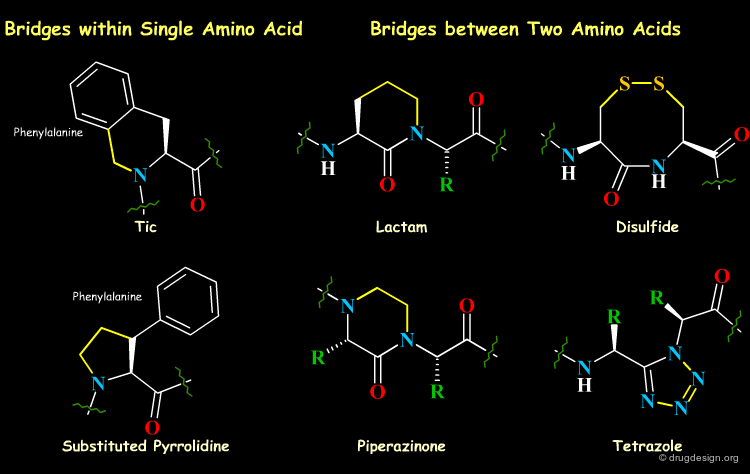
Short-Range Cyclizations (Bridging)¶
A successful strategy for the development of peptidomimetics involves the synthesis of conformationally restricted peptide analogs that imitate the receptor-bound conformation of the peptidic ligand as closely as possible, with the introduction of local bridges between different atoms in the molecule. The bridges, can be of various lengths and can link two side chain atoms, two backbone atoms, or a side chain atom and a backbone atom.
articles
Conformationally Restricted Peptides Through Short-Range Cyclizations Toniolo C Int. J. Pept. Protein Res. 35 1990
Conformationally Constrained Amino Acids. Synthesis and Optical Resolution of 3-Substituted Proline Derivatives Chung JYL, Wasicak JT, Arnold WA, May CS, Nadzan AM, and Holladay MW J. Org. Chem 55 1990
Synthesis of Chiral Piperazin-2-ones as Model Peptidomimetics DiMaio J and Belleau B J. Chem. Soc. Perkin Trans. 1 9 1989
Bioactive Conformation of Luteinizing Hormone-Releasing Hormone: Evidence from a Conformationally Constrained Analog. Freidinger RM, Veber DF, Perlow DS, Brooks JR and Saperstein R Science 210 1980
A New Approach to Receptor Ligand Design: Synthesis and Conformation of a New Class of Potent and Highly Selective Opioid Antagonists Utilizing Tetrahydroisoouinoline Carroxylic Acid Kazmierski W and Hruby VJ Tetrahedron 44 1988
A Molecular Constraint that Generates a cis Peptide Bond Sukumaran DK, Prorok M, and Lawrence DS J. Am. Chem. Soc. 113 1991
Conformational Mimicry. 1. 1,5-Disubstituted Tetrazole Ring as a Surrogate for the Cis Amide Bond Zabrocki J, Smith GD, Dunbar JB Jr., Iijima H, and Marshall GR J. Am. Chem. Soc. 110 1988
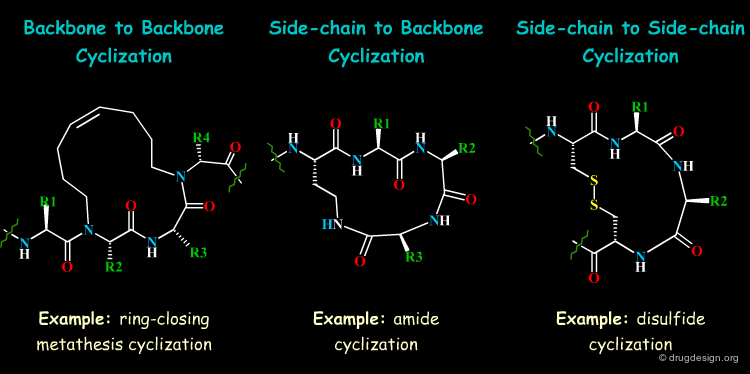
Long Range Cyclizations¶
Global restrictions of the peptide conformation are possible by limiting the flexibility of the peptide strand through cyclizations. In order to retain the biological activity, cyclic constraints should act on the backbone conformation to allow crucial side-chain interactions with the receptor to be strengthened.
articles
Emerging Approaches in the Molecular Design of Receptor-selective Peptide Ligands: Conformational, Topographical and Dynamic Considerations Hruby VJ, al-Obeidi F, and Kazmierski W Biochem. J. 268 1990
Backbone Cyclization: A New Method for Conferring Conformational Constraint on Peptides Gilon C, Halle D, Chorev M, Selinger Z, and Byk G. Biopolymers 31 1991
Cyclization Strategies in Peptide Derived Drug Design Li P and Roller PP Curr. Top. Med. Chem. 2 2002
Application of Ring-Closing Metathesis to the Synthesis of Rigidified Amino Acids and Peptides Miller SJ, Blackwell HE, and Grubbs RH J.Am. Chem. Soc. 118 1996
Synthesis of Cyclic Peptides by Ring-Closing Metathesis Reichwein JF, Versluis C, and Liskamp RMJ J.Org. Chem. 65 2000
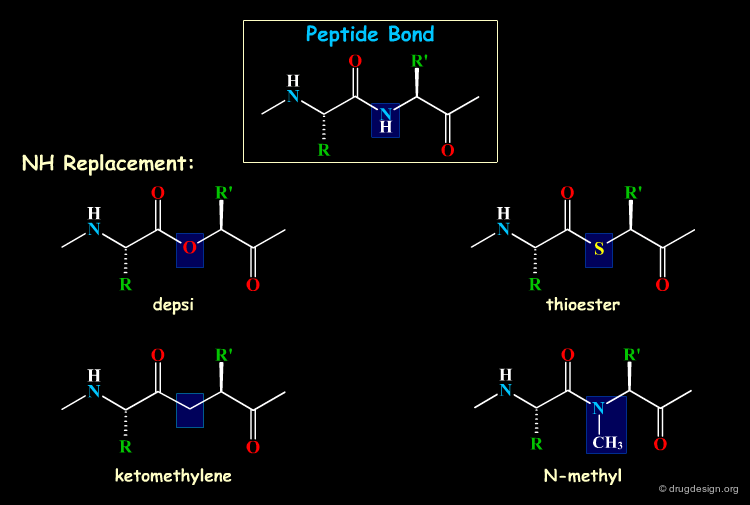
Mimicking the Peptidic Bond¶
Some atoms or group of atoms of the backbone of the peptide can be replaced by equivalent groups. The following examples are some of the most common replacements considered in backbone mimicry.
articles
Protease Inhibitors: Current Status and Future Prospects Leung D, Abbenante G and Fairlie DP J. Med. Chem. 43 2000
Structure-Based Inhibitors of HIV-1 Protease Wlodawer A and Erickson JW Annu. Rev. Biochem. 62 1993
book
Spatola AF Chemistry and Biochemistry of Amino Acids, Peptides and Proteins, Vol. III Marcel Dekker 1983
Browser of Bioisosteric Replacements¶
The following illustrates various chemical groups that can substitute for the peptide bond in a biomolecule.
Cα Modifications¶
Modification of the amino acid α-carbon is another approach of backbone mimicry. For example it is possible to replace the Cα with a nitrogen atom (to give azapeptides) or a boron (to give borapeptide), and also consider mono or di-substitutions of the α-carbon atom (synthetic non-coded amino acids).
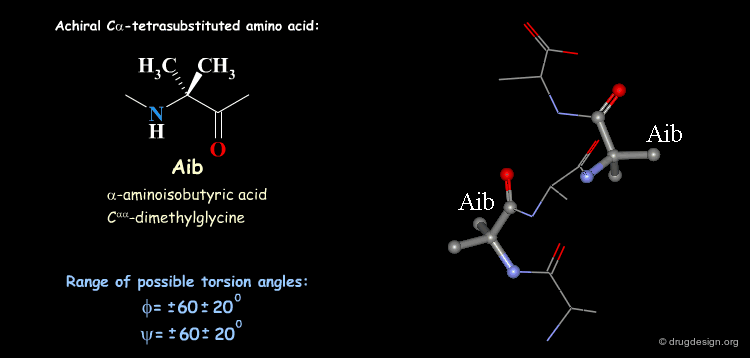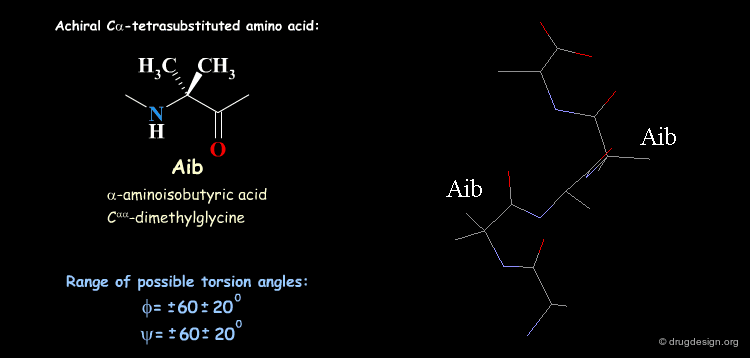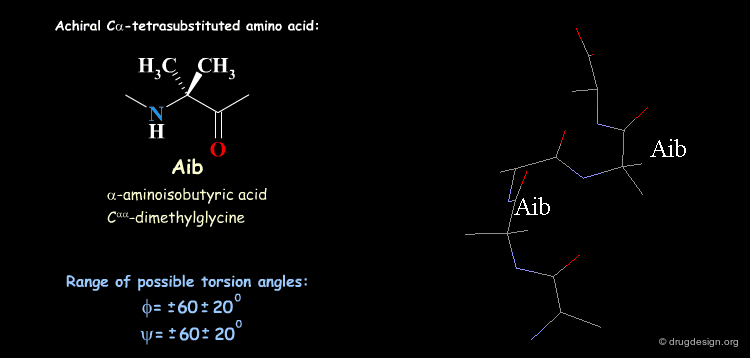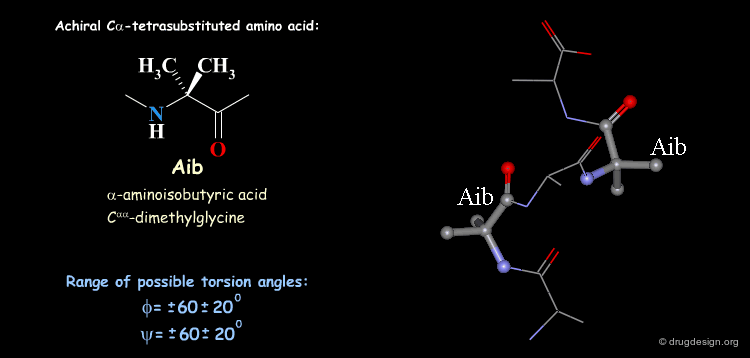
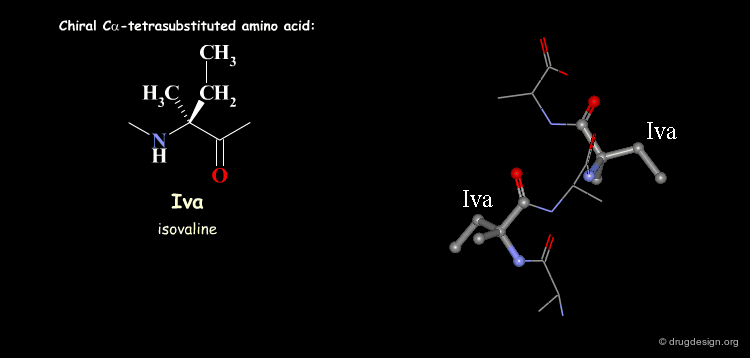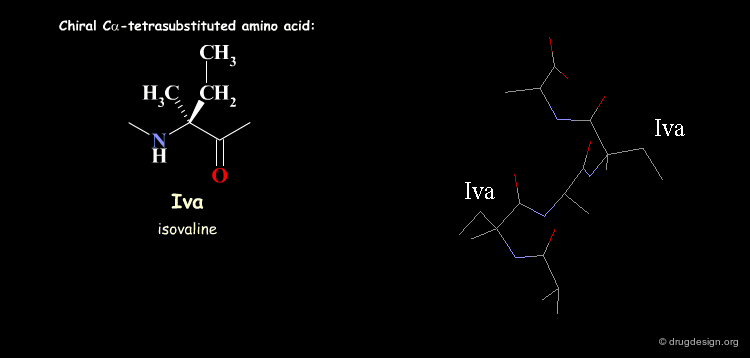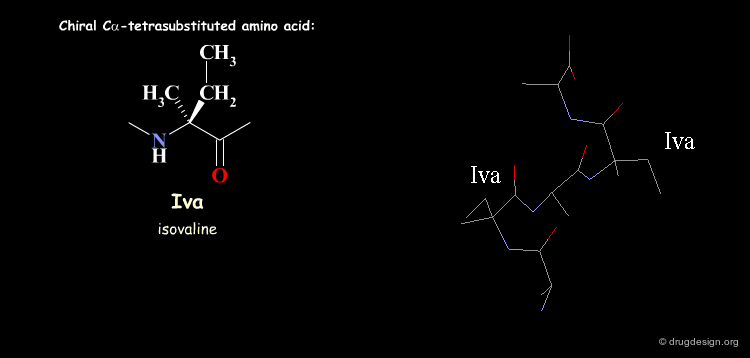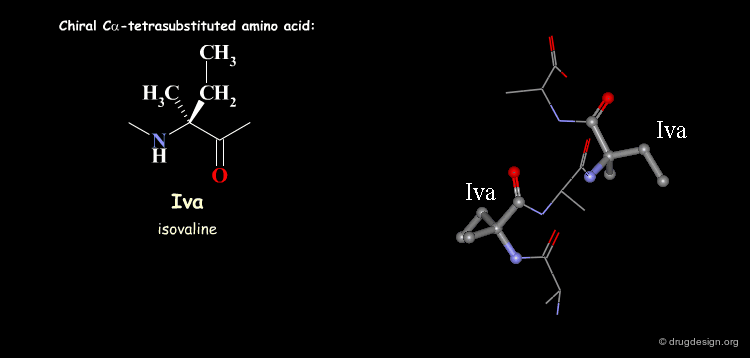
Tetra Substituted Amino Acids¶
Tetra substituted amino acids are widely used in peptidomimetics for their conformational properties that are restricted by steric effects with the substituents. When inserted into a peptide chain, most of the tetra substituted amino acids are strong promoters of folded and helical conformations such as β-turns, α-helices and 310-helices. Here we present some typical situations.
articles
Non-Standard Amino Acids in Peptide Design and Protein Engineering Balaram P Curr. Opin. Struct. Biol. 2 1992
Control of Peptide Conformation by the Thorpe-Ingold Effect (C-alpha Tetrasubstitution) Toniolo C, Crisma M, Formaggio F and Peggion C Biopolymers 60 2001
Azapeptides¶
Azapeptides have emerged as another important class of biologically stable and conformationally constrained backbone mimics. Aza amino acids provide a unique conformational property to the peptide backbone because of a chiral center loss (the α-carbon) and the extension of the area of planarity as compared with that of a normal amide bond. It was found that this more constrained backbone is limiting the peptide conformations to β-turn like conformations and helical structures.
articles
Conformational Properties of Azapeptides Thormann M and Hofmann H-J J. Mol. Struct.(Theochem) 469 1999
Azapeptidese Gante J Synthesis 1989(6) 1989
Extension of the Peptide Chain¶
The extension of the peptide backbone is another way to make a peptide mimic which is resistant to proteolytic enzymes. An extended backbone structure introduces additional degrees of torsional freedom as compared to the two degrees of the α-amino acids. However, the conformational space of the combined torsion angles is quite limited and thus, well conceived oligomers can adopt well-defined secondary structures such as helices, turns, and small sheet like structures. Such oligomers and other polymers that tend to adopt specific compact conformations are known as foldamers.
articles
Foldamers: A Manifesto Gellman SH Acc. Chem. Res. 31 1998
Designing Polymers that Mimic Biomolecules Kirshenbaum K, Zuckermann RN and Dill KA Curr. Opin. Struct. Biol. 9 1999
beta-Peptides: A Surprise at Every Turn Seebach D and Matthews JL Chem. Commun.
1997
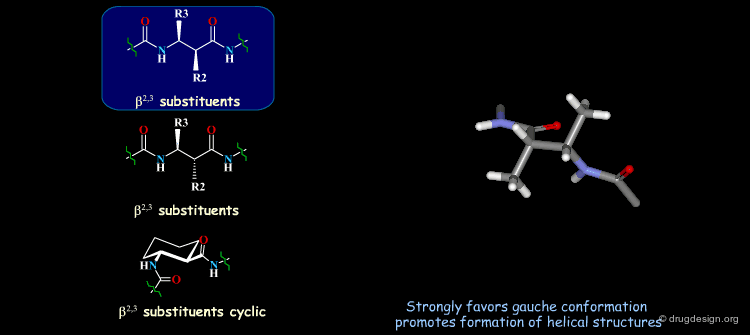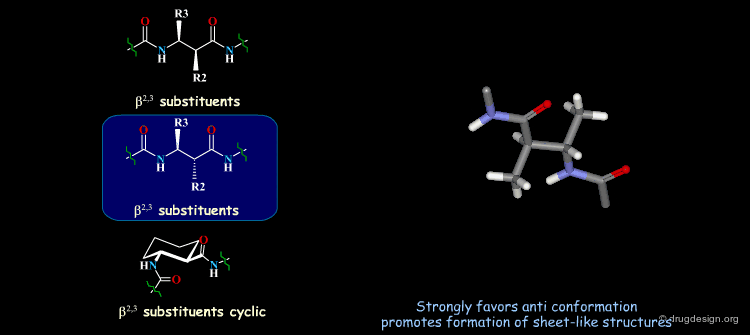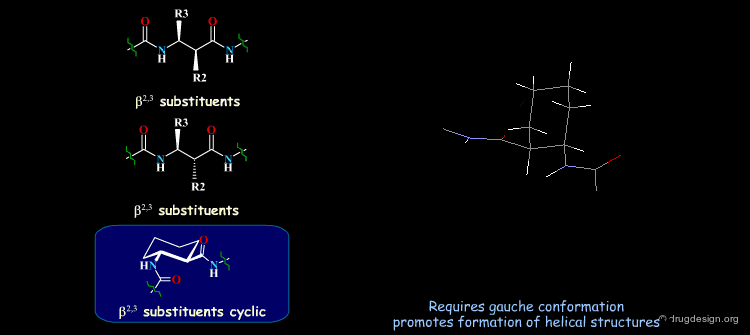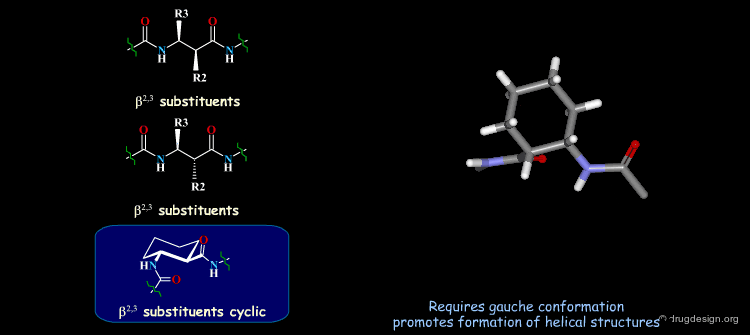
β-Peptides¶
β-peptides are oligomers build of β-amino acids, which include one extra carbon in the backbone unit. The effects of substituents on the conformation of β-amino acids have been the subject of extensive experimental and theoretical studies and are here summarized. In general, a gauche conformation around the Θ torsion angle of the C2-C3 bond (see figure) will lead to helical or turn-like structures of the β-peptides whereas a trans conformation will lead to a sheet-like structure.
articles
beta-Peptides: From Structure to Function Cheng RP, Gellman H, and DeGrado WF Chem. Rev. 101 2001
The (P)-28-Helix of a beta-Hexapeptide Consisting of (2R, 3S)-3-Amino-2-hydroxy Acid Residues Gademann K, Hane A, Rueping M, Jaun B,and Seebach D Angew. Chem. Int. Ed 42 2003
Foldamers: A Manifesto Gellman SH Acc. Chem. Res. 31 1998
Probing the Helical Secondary Structure of Short-Chain [beta]-Peptides Seebach D, Ciceri PE, Overhand M, Jaun B, Rigo D, Oberer L, Hommel U, Amstutz R, and Widmer H Helv. Chim. Acta 79 1996
beta-Peptides: A Surprise at Every Turn Seebach D and Matthews JL Chem. Commun.
1997
beta-Amino Acids: Versatile Peptidomimetics Steer DL, Lew RA, Perlmutter P, Smith AI and Aguilar M-I Curr. Med. Chem. 9 2002
Problems with Peptide-Based Analogs¶
The main problem in the first route (successive modifications of a reference peptide) is to know when to stop the process. The following pages illustrate typical difficulties encountered in the development of this approach.
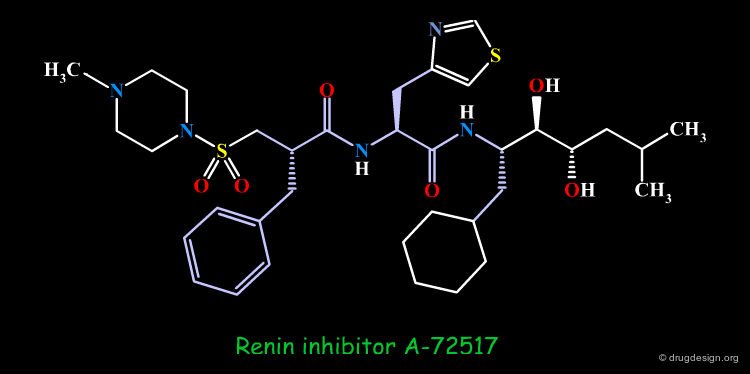
Example of Reduction of Peptide Character¶
The peptidic sequence of angiotensinogen (the substrate of renin) served as a basis for the discovery of renin inhibitors. A-72517 (Zankiren) is an example of a very potent inhibitor with a substantially reduced peptidic character.
articles
Novel Renin Inhibitors Containing the Amino Acid Statine Boger J, Lohr NS, Ulm EH, Poe M, Blaine EH, Fanelli GM, Lin TY, Payne LS, Schorn TW, LaMont BI, Vassil TC, Stabilito II, Veber DF, Rich DH and Bopari AS Nature 303 1983
Renin Inhibitors Greenlee WJ Med. Res. Rev. 10 1990
Analysis of Structure-Activity Relationships in Renin Substrate Analogue Inhibitory Peptides Hui KY, Carlson WD, Bernatowicz MS and Haber E J Med. Chem. 30 1987
Orally Potent Human Renin Inhibitors Derived from Angiotensinogen Transition State: Design, Synthesis, and Mode of Interaction Iizuka K, Kamijo T, Harada H, Akahane K, Kubota T, Umeyama H, Ishida T and Kiso Y J. Med. Chem. 33 1990
Discovery of a Peptide-based Renin Inhibitor with Oral Bioavailability and Efficacy Kleinert HD, Rosenberg SH, Baker WR, Stein HH, Klinghofer V, Barlow J, Spina K, Polakowski J, Kovar P, Cohen J and Denissen J Science 257 1992
In Search of Ideal Antihypertensive Drugs: Progress in Five Decades Lien EJ, Gao H and Lien LL Prog Drug Res. 43 1994
Design and Synthesis of Renin Inhibitors: Incorporation of Transition-state Isostere Side Chains That Span from the S1 to the S3 Binding Pockets and Examination of P3-Modified Renin Inhibitors Plummer MS, Shahripour A, Kaltenbronn JS, Lunney EA, Steinbaugh BA, Hamby JM, Hamilton HW, Sawyer TK, Humblet C, Doherty AM, Taylor MD, Hingorani G, Batley B and Rapundalo ST J. Med. Chem. 38 1995
Potent New Inhibitors of Human Renin Szelke M, Leckie B, Hallett A, Jones DM, Sueiras J, Atrash B and Lever AF Nature 299 1982
Inhibitors of Renin as Potential Therapeutic Agents Wood JM, Stanton JL and Hofbauer KG J. Enzyme. Inhib. 1 1987
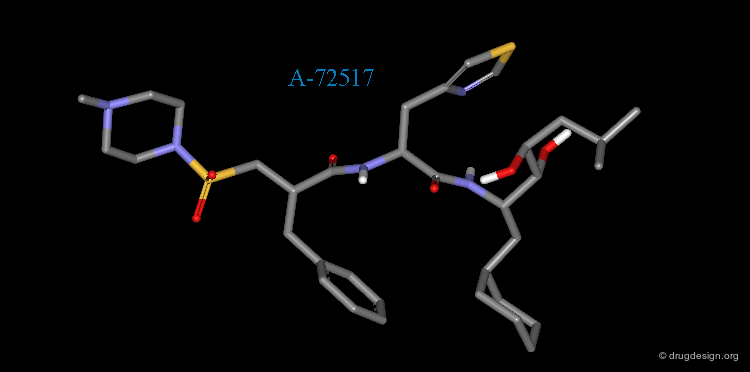
A-72517 is a Mimic of Angiotensinogen¶
The superimposition of A-72517 with the reference peptide is displayed below and shows how the designed structure alligns well in 3D with the amino-acids adjacent to the scissile bond of angiotensinogen (center).
Dead End in the Development of A-72517¶
Although very active in vitro, the clinical development of A-72517 was hindered by poor pharmacokinetics, which included low oral bioavailability and rapid excretion. The clinical trials on the renin inhibitor A-72517 were discontinued; Zankiren did not reach the market. The "depeptidization" operation require to be further pursued.
Second Route:De Novo Design of Non Peptide Mimics¶
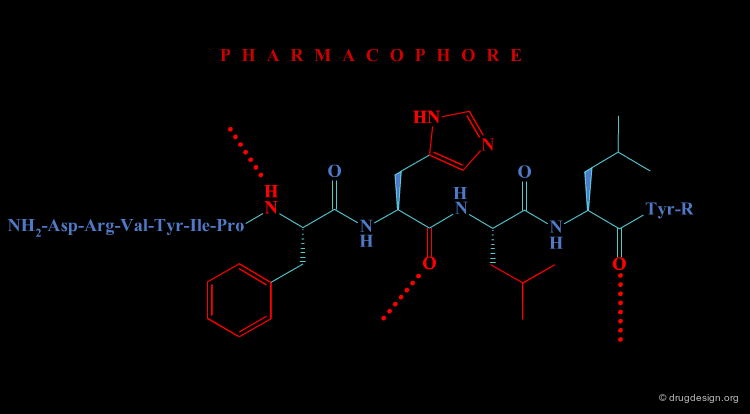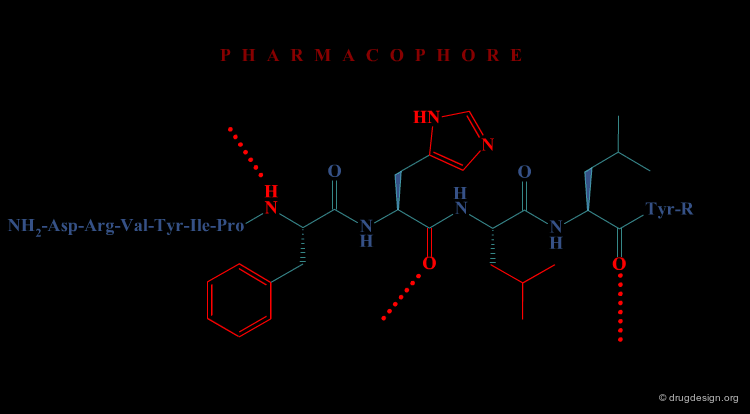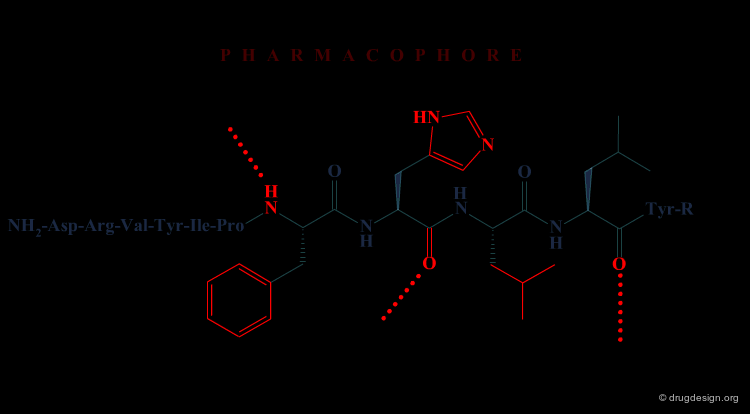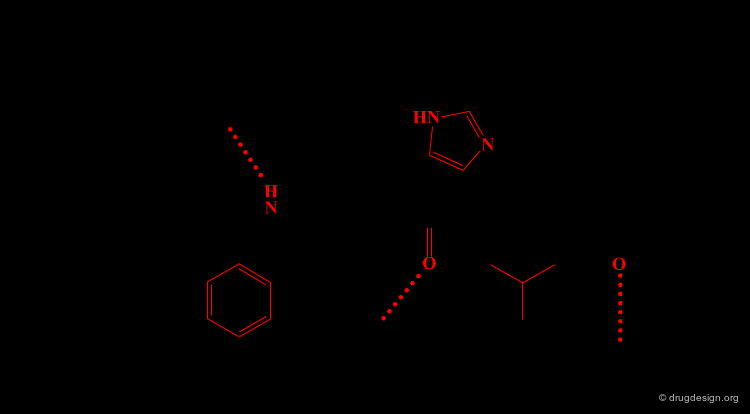
In the second approach, the activity of the initial peptide is viewed as created by a favorable 3D arrangement of pharmacophore elements. This information will be the basis of a "de novo" design of chemically unrelated substances mimicking in 3D the important moieties of the reference peptide.
Operational Framework¶
The activity of a molecule is due to its proper 3D pharmacophoric arrangement. This method requires data of high informational content that will be used for the identification of the pharmacophoric moieties.
Removing Non-Essential Fragments¶
In the second phase, and based on the previous analysis all non-essential structural fragments are removed from the reference peptide. This provides an operational framework in which the peptidic elements are removed.
Design of New Molecules¶
The operational framework previously defined allows the design to proceed. In this phase, new molecules carrying the proper 3D pharmacophoric elements are designed.
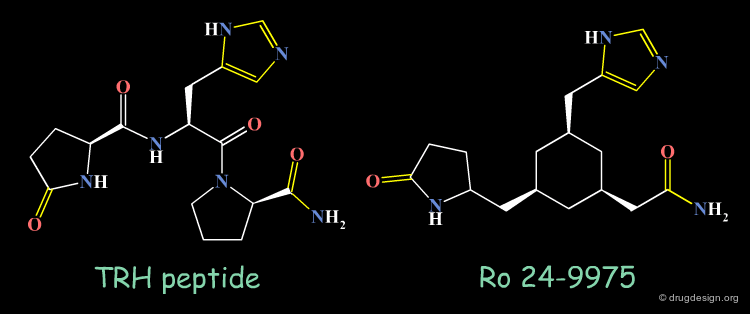
Thyrotropin-Releasing Hormone (TRH) Example¶
TRH (Thyrotropin-Releasing Hormone) is produced by the hypothalamus and this peptide has been considered as a target for treating Alzheimer disease and spinal injury.
articles
Design of Nonpeptides from Peptide Ligands for Peptide Receptors Hruby VJ, Qui W, Okayama T and Soloshonok VA Methods Enzymol 343 2002
Concepts and Progress in the Development of Peptide Mimetics Olson GL, Bolin DR, Bonner MP, Bos M, Cook CM, Fry DC, Graves BJ, Hatada M, Hill DE, Kahn M, Madison VS, Rusiecki VK, Sarabu R, Sepinwall J, Vincent GP and Voss ME J. Med. Chem. 36 1993
Peptide Mimetics of Thyrotropin-releasing Hormone Based on a Cyclohexane Framework: Design, Synthesis, and Cognition-enhancing Properties Olson GL, Cheung HC, Chiang E, Madison VS, Sepinwall J, Vincent GP, Winokur A and Gary KA J. Med. Chem. 38 1995
Ro-24-9975 is a Non-Peptidic Mimic of TRH¶
Design effort for identifying non-peptidic mimics of TRH succeeded in creating molecules such as Ro 24-9975 as potential drug candidates. The X-ray structure of TRH, NMR studies and molecular modeling were essential parts in the discovery of such mimics. An illustration of the concept considered in this projet is given in the following page.
articles
Peptide Mimetics of Thyrotropin-releasing Hormone Based on a Cyclohexane Framework: Design, Synthesis, and Cognition-enhancing Properties Olson GL, Cheung HC, Chiang E, Madison VS, Sepinwall J, Vincent GP, Winokur A and Gary KA J. Med. Chem. 38 1995
Conformational Properties of Central Nervous System Active Thyrotropin Releasing Hormone Analogues: Probing Structure-activity Relationships at the Molecular Level Eckle E and Stezowski JJ J. Med. Chem. 28 1985
Molecular Conformation of Thyrotropin Releasing Hormone from the X-ray Analysis of its Tartrate Kamiya K, Takamoto M, Wada Y, Fujino M and Nishikawa M J. Chem. Soc. Chem. Commun. 438-9 1980
Which Route Should be Used?¶
The first approach is conceptually straightforward whereas the second is more complicated to implement. In both cases it is difficult to estimate the amount of effort that will be necessary to obtain compounds satisfying the criteria of good oral activity and pharmaco-kinetic properties. Examples of both approaches will be extensively presented and discussed in the following chapter.
The Challenge of Peptidomimicry¶
Challenges in Peptide Modifications¶
The successive modifications of the structure of a reference peptide are feasible. The advantages of this approach are multiple: a number of biologically active molecules are generated; they shed light on the structural requirements for biological activities; and they pave the road for deciding which molecules to synthesize next. This approach requires however a continuous effort of chemical syntheses. The accumulation of active molecules may nurture the illusion that the breakthrough is "just around the bend". However in this approach it is difficult to assess if substantial progress was made until real clinical trials indicate the exact situation. If it succeeds, the knowledge gained can be easily transposed to other similar projects. If it fails, the structural information acquired is of great value for considering a de novo design strategy for non peptidic mimics.
Challenges in Non-Peptidic Mimicry¶
The de novo design strategy for non-peptidic mimics is an alternative approach for utilizing the information acquired on the reference peptide. When a non-peptidic compound is discovered, its scaffold provides the necessary framework to optimize specificity, oral bioavailability and pharmacokinetic properties. Advanced computerized molecular design technologies are useful for exploiting optimally the information available and for the design and the synthetic efforts that have to be developed in an integrated manner. Sufficient time might be needed before the breakthrough occurs and patience is needed when inactive molecules are obtained.
Perspectives in Peptidomimetics¶
There is no systematic method for transforming a peptide into a substance for drug development. In the past, medicinal chemists tended to embark on the process of making analogs of the peptide rather rapidly, hoping to find within a reasonable amount of time a compound with entirely different pharmacokinetic properties.
Today since many success stories on de novo peptidomimicry have been reported, this approach has become more accepted. Currently, this trend encourages the development of sophisticated computerized packages allowing the enormous amount of experimental data accumulated in the course of many projects to be exploited in a global way.
This encourages the creation of entirely new conditions for drug design and the definition of an entirely new way of considering lead discovery.
Copyright © 2024 drugdesign.org