Case Studies in Peptidomimetics¶
Info
Several examples of peptidomimetic approaches are described and discussed. The exploitation of the structure of a reference peptide molecule to obtain a non-peptidic mimic is described for each of them.
Number of Pages: 76 (±1 hours read)
Last Modified: January 2009
Prerequisites: Introduction to Peptidomimetics
Case Study-1 : Somatostatin Mimicry¶
Somatostatin Structure¶
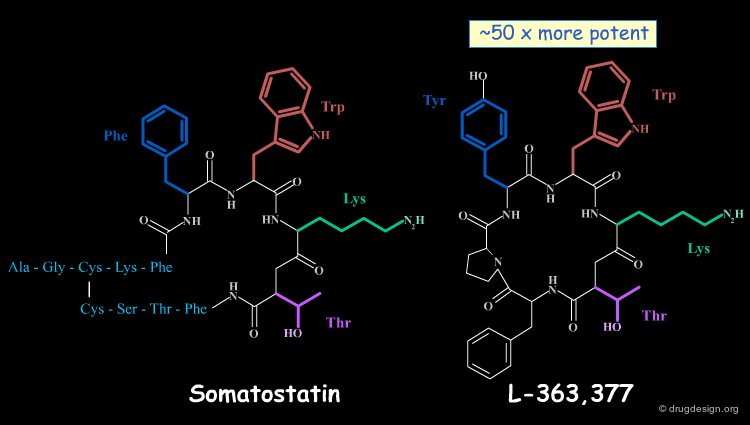
Somatostatin (also known as somatotropin release-inhibiting factor, SRIF) is a cyclic peptide hormone that was discovered in 1973; it is sometimes considered to function as a neurotransmitter. In the following pages we present attempts that were made to discover smaller and non-peptidic molecules having somatostatin-like biological activities.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Somatostatin Receptors¶
The somatostatin receptors belong to the family of seven-transmembrane G-protein coupled receptors and constitute an important target in drug discovery. The potential therapeutic applications principally involve the treatment of diabetes (reduced gluconeogenesis by inhibition of insulin and glucagon release), treatment of cancers, but also the treatment of acromegaly (by inhibition of growth hormone release) and ulcers and related gastrointestinal disorders (by inhibition of gastric acid secretion).
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Subtypes of Somatostatin Receptors¶
Five subtypes of somatostatin receptors are known (sstr1...sstr5). A model of the 3D structure of one of them is shown in the following view (model constructed with the program "WHAT IF" by homology with the X-ray structure of bacteriorhodopsin).
The Drug Discovery Strategy¶
The short duration of somatostatin action limits its use as a drug and has prompted the search for analogs with greater metabolic stability. Since the exact interactions between somatostatin and its receptor are not known, the drug discovery strategy concentrated solely on mimicking the somatostatin structure. The concept used in this approach is outlined in the view below (example taken from substance P mimicry).
The Somatostatin Pharmacophore¶
It was the Salk group who first showed that the complete somatostatin molecule is not necessary for full activity. This prompted other groups such as Merck and Sandoz to analyze the structure-activity relationships (SAR) in terms of the geometrical conformations of the molecules. Merck proposed a model of the bioactive conformation of somatostatin and also suggested that the minimal peptide sequence necessary for activating the receptor consisted of the Phe7-Trp8-Lys9-Thr10 tetra-peptide sequence.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Successful Reduction of the Somatostatin¶
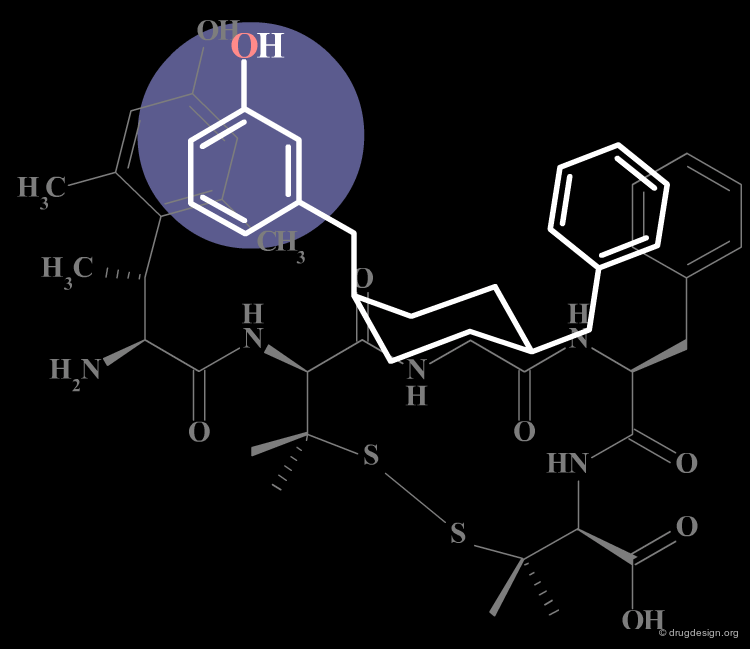
Conformationally constrained peptides that proved to be several times more potent and more selective than somatostatin were found. The cyclic hexapeptide compound L-363,377 is an example of the successful reduction of the somatostatin structure that carries the desired structural requirements. L-363,377 is about 50 times more potent than somatostatin in inhibiting insulin secretion.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
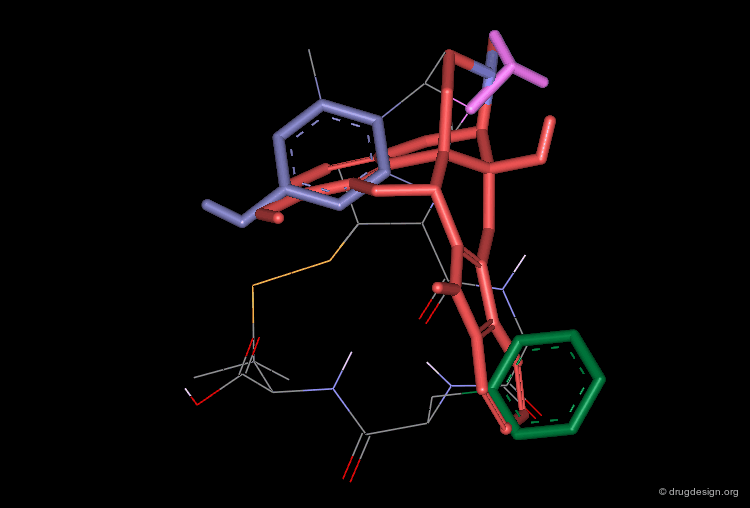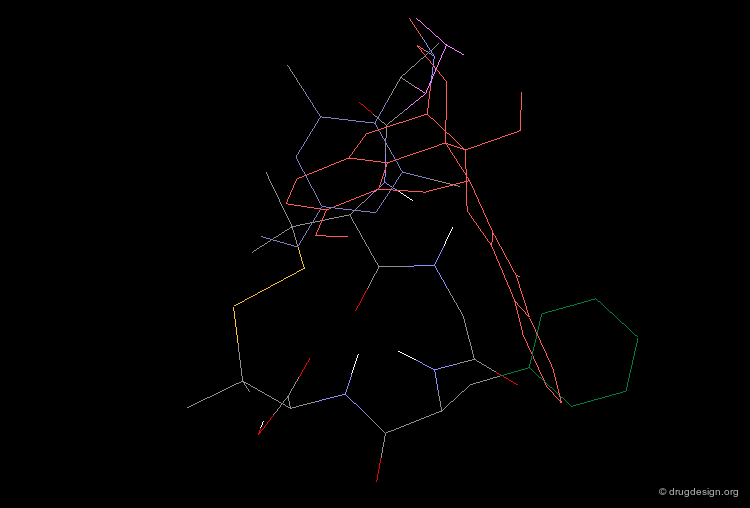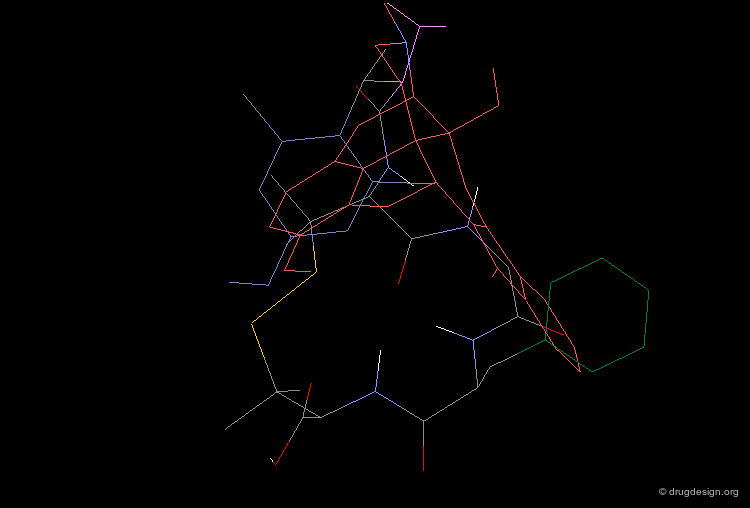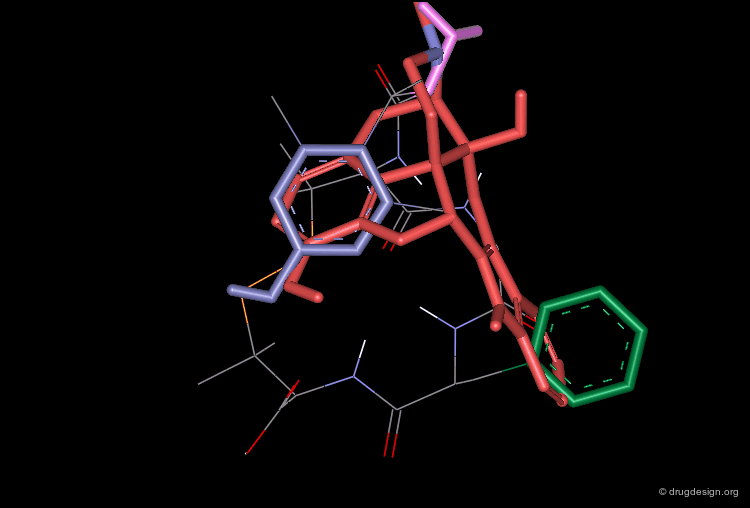
Mimics of L-363,377 with Database Searching¶
The Merck group used the structure of L-363,377 as a query to search in their own corporate chemical database for analogs. At this time the Merck database contained about 200,000 compounds and the search was oriented for a fit with the Tyr-Trp-Lys pharmacophore of the cyclic peptide.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Results of the Database Searching¶
Based on the similarity with this pharmacophore, seventy-five compounds were selected to be tested biologically and L-264,930 is one of them. The compound was found to have an IC50 of 100 nM for the human sstr2 receptor and considered interesting because of its reduced peptidic character.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
A Good Mimic of the Reference Cyclic Peptide¶
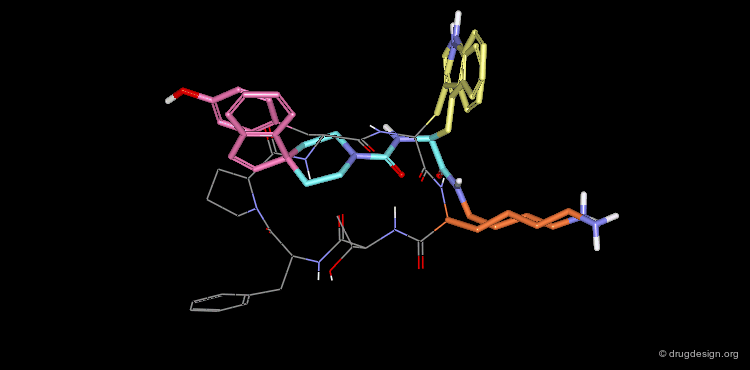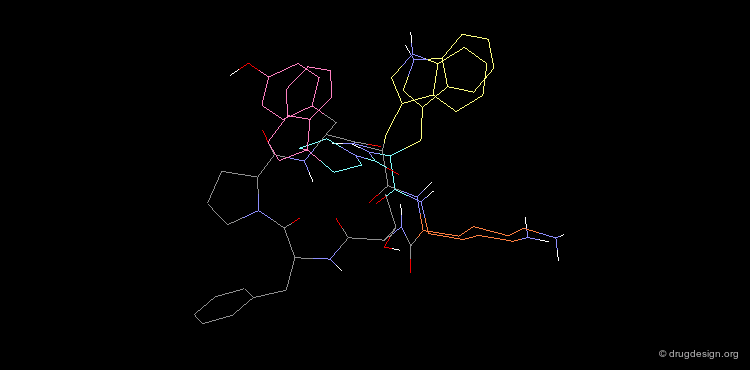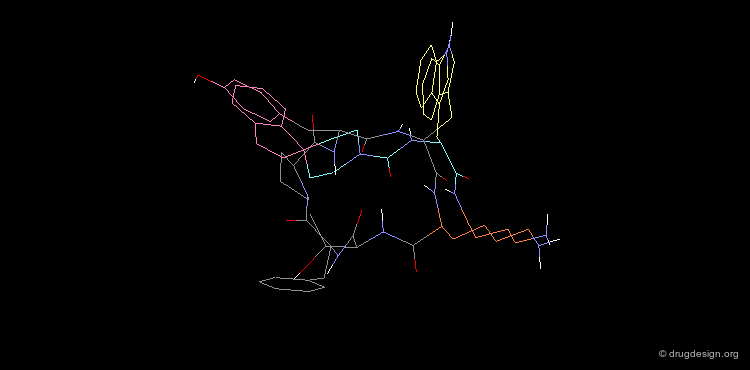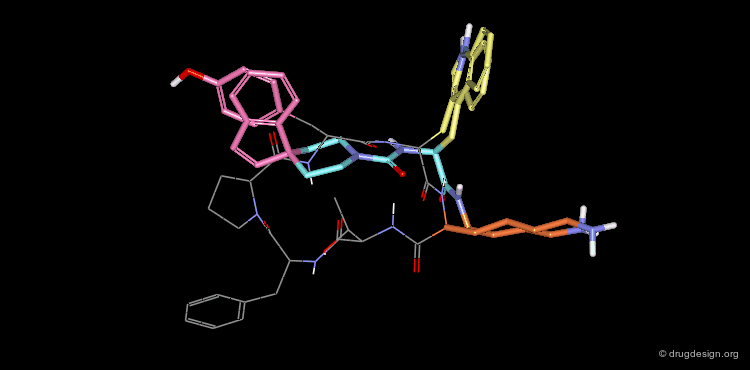
The superimposition of the two compounds in 3D allows us to understand why the non-peptidic molecule L-264,930 is a good mimic of the reference cyclic peptide L-263,377. As illustrated in the following view, the 3D alignment of the two molecules reveals a good overlap of the tryptophan (Trp), the lysine (Lys) and the tyrosine (Tyr) amino-acid side chains of L-263,377 with corresponding similar fragments of L-264,930.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Development of a Combinatorial Chemistry Approach¶
The L-264,930 scaffold proved to be suitable for the development of a combinatorial chemistry approach. 79 substituents were considered for R1, 20 for R2 and 20 for R3, defining a virtual library of 79x20x20 analogs, which were synthesized and screened for all the five somatostatin sub-classes of receptors.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
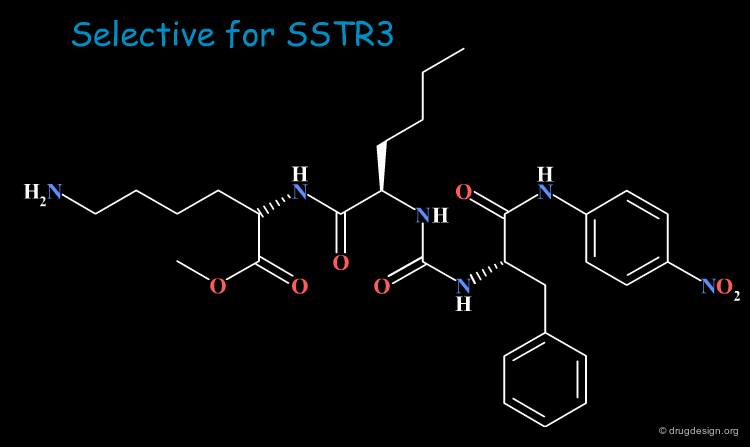
Combinatorial Chemistry Results¶
SAR analyses led to the further refinement and design of a more focused library. This effort enabled the discovery of various potent and selective analogs; some examples are shown in the following view.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
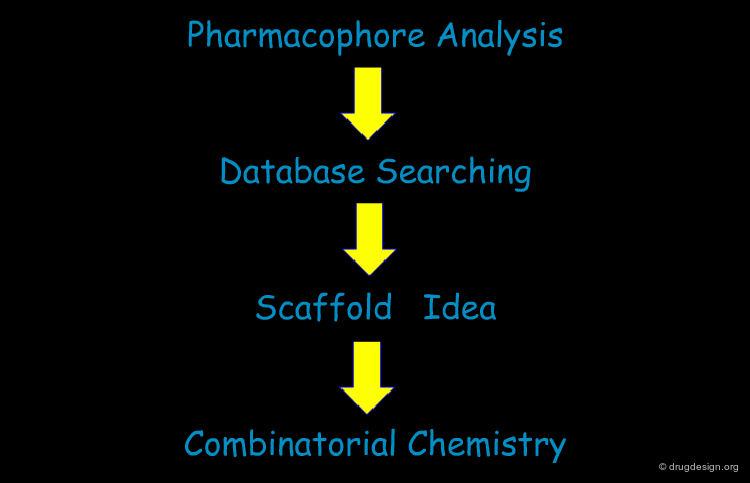
An Integrated Approach to Drug Discovery¶
The somatostatin story illustrates the good exploitation of the complementary aspects of different approaches. In a peptidomimetics perspective the pharmacophore analyses, database searching and library design were combined in order to discover potent and selective non-peptidic agonists for each of the five sub-classes of somatostatin receptors. The key to the success was the correct initial analyses of the pharmacophore requirements. This allowed for the successful deployment of a database searching approach to reveal a new and non-peptidic scaffold. Combinatorial chemistry then contributed to the systematic exploration of the stereochemical features of the various receptors, which resulted in the discovery of potent and specific ligands of great potential.
articles
Synthesis and Biological Activities of Potent Peptidomimetics Selective for Somatostatin Receptor Subtype 2 Yang L, Berk SC, Rohrer SP, Mosley RT, Guo L, Underwood DJ, Arison BH, Birzin ET, Hayes EC, Mitra SW, Parmar RM, Cheng K, Wu TJ, Butler BS, Foor F, Pasternak A, Pan Y, Silva M, Freidinger RM, Smith RG, Chapman K, Schaeffer JM and Patchett AA. Proc. Natl. Acad. Sci. USA 95 1998
Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT and Schaeffer JM. Science 282 1998
Synthesis and Biological Activity of Highly Potent Heptapeptide Analogs of Somatostatin with C-Terminal Modifications Janecka A and Zubrzycka M Endocrine Regulations 35 2001
Case Study-2 : δ-Opioid Receptor Agonists¶
Therapeutic utility of δ-Opioid Receptor Agonists¶
Selective agonists of the δ-opioid receptor are of great interest being expected to lead to analgesic agents with no adverse side effects such as the development of physical dependence or respiratory depression.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Typical Peptide δ-Opioid Receptor Agonists¶
The structures of some typical δ-opioid receptor peptide agonists are shown here. Some non-peptidic agonists are also known (see next page). Here we present how the known data enabled scientists to design and discover an entirely new and nano-molar potent non-peptide agonist.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Typical Non-Peptide δ-Opioid Receptor Agonists¶
Several non-peptide ligands of the δ-opioid receptor such as SIOM, OMI and TAN-67 have been discovered either by modification of morphine-type alkaloids, or by random screening. However such compounds are not enough selective and trigger additional unwanted biological activities.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Pharmacophore for δ-Opioid Receptor Agonists¶
Precise SAR studies have shown that the structural elements that are essential for peptide ligands to recognize the δ-opioid receptor and produce physiological effects are the following:
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
SAR, NMR and Modeling of the DPDPE series (1/3)¶
X-Ray crystallography and NMR analyses of DPDPE indicate that the conformations of the 14-membered ring, in solution and in the crystal form, are very close.
articles
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
SAR, NMR and Modeling of the DPDPE series (2/3)¶
The substitution of the Phe-4 residue with the four isomers of β-methyl-phenylalanine, followed by NMR and molecular modeling studies led to the conclusion that the interaction of Phe-4 with the δ-opioid receptor requires a Gauche (-) conformation for the side-chain of this residue.
articles
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
SAR, NMR and Modeling of the DPDPE series (3/3)¶
Similar analyses for the Tyr-1 residue indicate that the Anti side-chain conformation of this residue is preferred for the interaction with the δ-opioid receptor.
articles
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Bioactive Conformation of DPDPE (1/4)¶
The probable bioactive conformation of DPDPE can be deduced on the basis of the preceding studies. As shown in the following view, this conformation aligns well with the molecular geometry of SIOM (shown here in yellow).
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Bioactive Conformation of DPDPE (2/4)¶
... and also with the molecular geometry of TAN-67 (shown here in purple).
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Bioactive Conformation of DPDPE (3/4)¶
... and also with the molecular geometry of OMI (shown here in red).
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Bioactive Conformation of DPDPE (4/4)¶
The probable bioactive conformation of DPDPE is represented in the view below with the three structural elements that were shown to be essential to recognize the δ-opioid receptor: the phenol ring, the amino group and the tyrosine residue. In this structure a distance of about 7 Å separate the two phenyl rings of the Phe-4 and the Tyr-1 moieties.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Scaffold Design of Non-Peptide Antagonists¶
A cyclohexane scaffold with two cis 1,4 substituents was imagined as a candidate template for mimicking the 7 Å distance unraveling the phenyl rings of Phe-4 and Tyr-1.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Refinement of the Scaffold and Substituents¶
Refinement of the scaffold shows that for mimicking the phenol moiety of Tyr-1, the hydroxyl group should be in meta.
Amino Group not Included¶
An amino group may be introduced on the cyclohexane scaffold to overlap the corresponding amino group of the DPDPE structure. However the first molecules synthesized did not include this amino group.
Reducing the Number of Chiral Centers¶
To facilitate the chemical syntheses and reduce the number of chiral centers, the two substituted carbon atoms of the cyclohexane template were replaced by nitrogen atoms, leading to a 1,4 piperazine six-membered ring scaffold for the first generation of such peptidomimetics.
Substituent with Variable Hydrophobicity¶
Finally, tyrosine analogs with greater hydrophobicity showed in DPDPE enhanced contribution to selectivity and potency. A substituent with variable hydrophobicity was therefore added in the designed template considered for the design of new molecules.
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
The First Series Synthesized¶
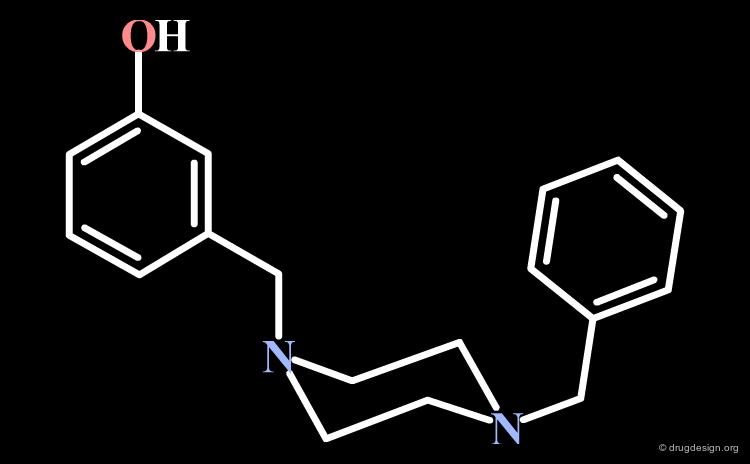
From the first analogs that were synthesized, SL-3111 emerged as a very potent δ-opioid receptor agonist with an IC50 of 8.4 nM. Moreover it is one of the most selective non-peptide ligands reported so far for binding to the δ-opioid receptor, in preference to the µ-opioid receptor (a 2000 fold selectivity).
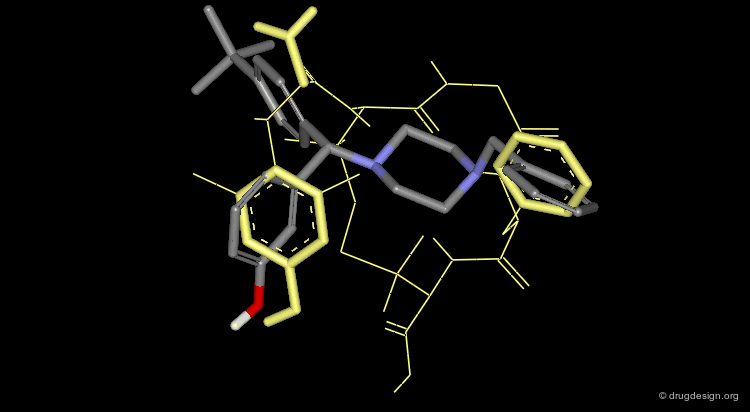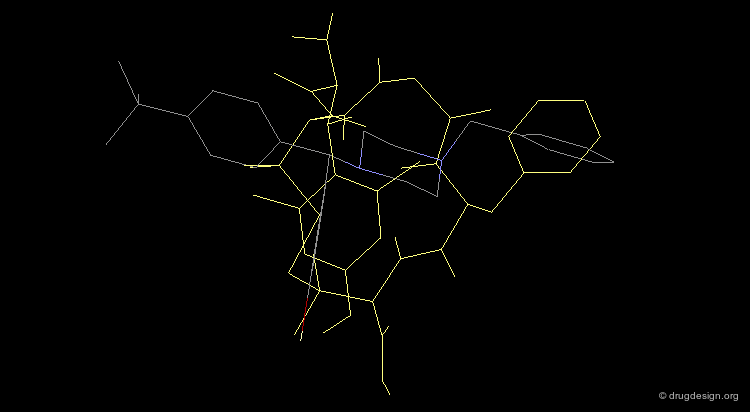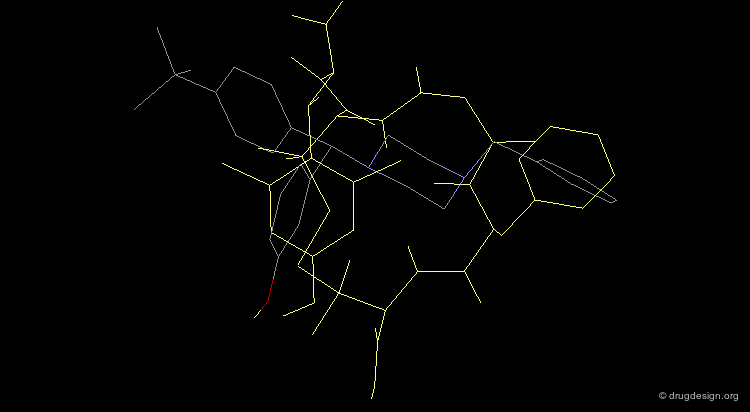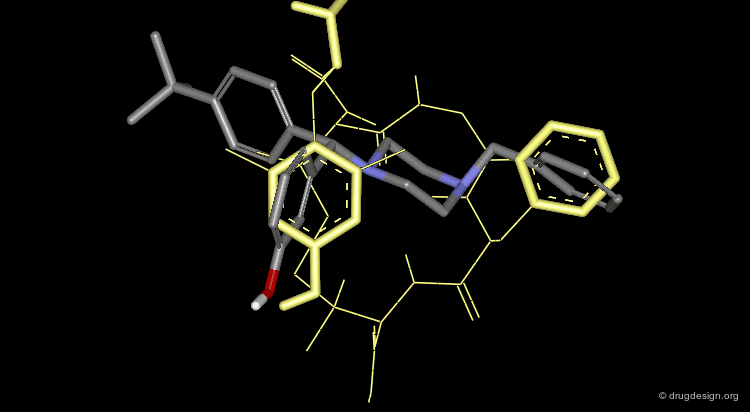
The (-) SL-3111 Enantiomer¶
The (-) SL-3111 enantiomer has a very potent binding affinity for the δ-opioid receptor (IC50 = 4.1 nM), an activity that can be understood by aligning this molecule (colored with atom types) with the bioactive conformation identified for DPDPE (colored in yellow).
articles
De Novo Design, Synthesis, and Biological Activities of High-Affinity and Selective Non-Peptide Agonists of the δ-Opioid Receptor Liao S, Alfaro-Lopez J, Shenderovich MD, Hosohata K, Lin J, Li X, Stropova D, Davis P, Jernigan KA, Porreca F, Yamamura HI and Hruby VJ Journal of Medicinal Chemistry 41 1998
A Three-Dimensional Model of the d-Opioid Pharmacophore: Comparative Molecular Modeling of Peptide and Nonpeptide Ligands Shenderovich MD, Liao S, Qian X and Hruby VJ Biopolymers 53 2000
X-ray Structure of [D-Pen2,D-Pen5]enkephalin, a Highly Potent, d Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations Flippen_Anderson JL, Hurby VJ, Collins N, George C and Cudney B Journal of the American Chemical Society 116 1994
Case Study-3 : MC4R Melanocortin Receptor Agonists¶
Melanocortin Receptors¶
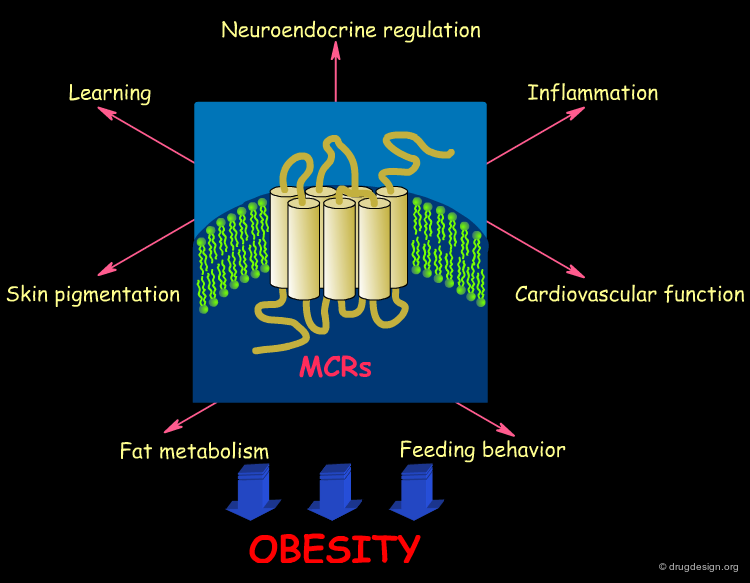
The melanocortin receptors (MCRs) are a family of seven-transmembrane G-protein coupled receptors that mediate many biological activities including neuroendocrine regulation, fat metabolism and feeding behavior. Agonists of the melanocortin subtype-4 receptor (MC4R) have been shown to promote satiety. This subclass of receptors is an attractive target for developing new therapies for obesity. In the following pages we discuss attempts to discover novel ligands (peptides and non-peptides) of the melanocortin receptor.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Minimal Peptide Sequence for Activating the Receptor¶
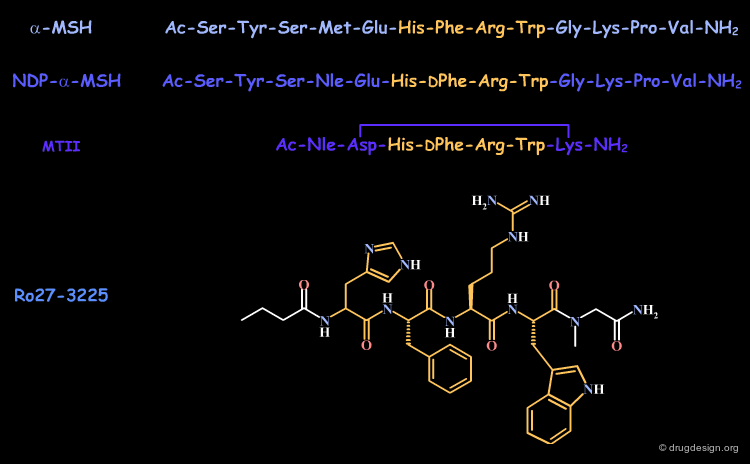
All the endogenous agonists (as for example α-MSH) contain the tetra-peptide sequence His-Phe-Arg-Trp considered to be the minimal peptide sequence necessary for activating the receptor. Further studies with MCR agonists have shown that phenylalanine can be replaced with its D-stereoisomer with greater agonistic activity.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Strategy for the Design of New Agonists¶
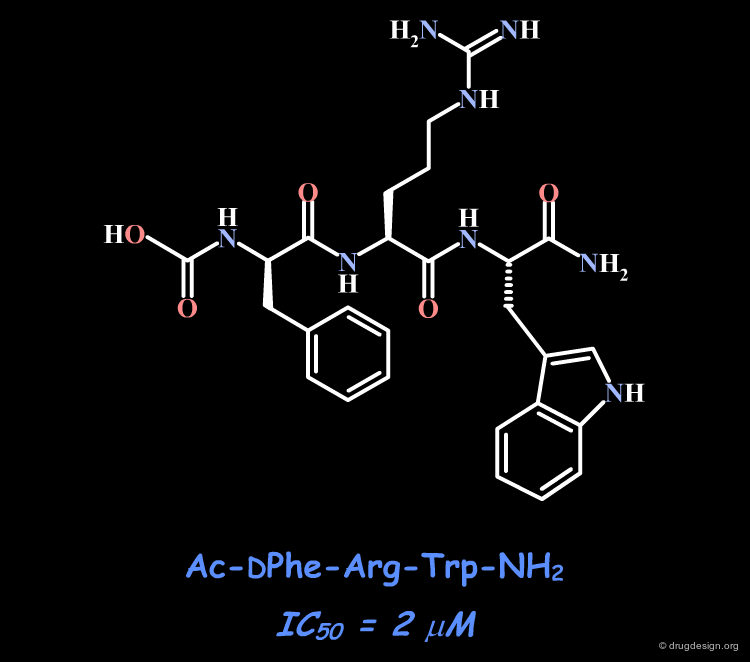
The observation that histidine can be replaced with alanine suggests a strategy aimed at mimicking the bioactive conformation of the tripeptide DPhe-Arg-Trp. The tripeptide alone is only weakly active.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Analogs of MTII, Lactam Derivatives of α-Melanotropin, Modified at the N-Terminus, and Their Selectivity at Human Melanocortin Receptors 3, 4, and 5 Bednarek MA, Macneil T, Kalyani RN, Tang R, Van der Ploeg LHT and Weinberg DH Biochemical and Biophysical Research Communications 261 1999
Discovery of Prototype Peptidomimetic Agonists at the Human Melanocortin Receptors MC1R and MC4R Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C and Gantzr I J. Med. Chem 40 1997
Cyclic Peptide 1¶
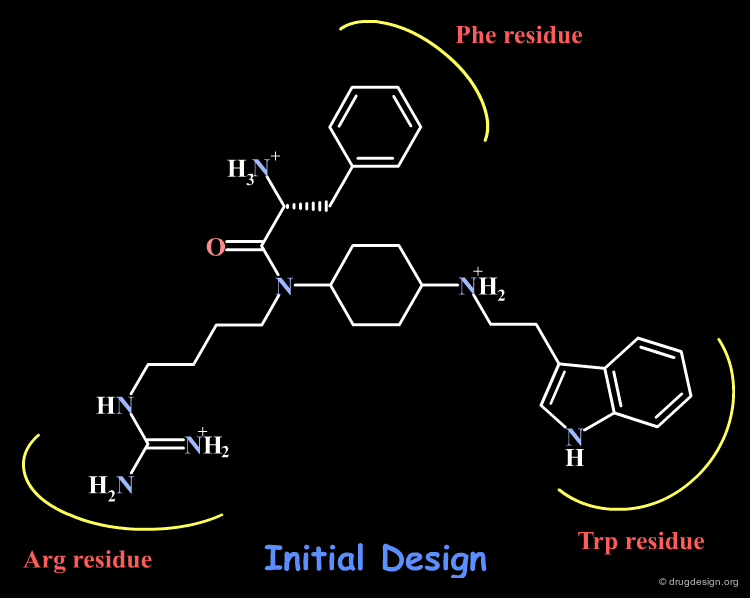
However the cyclic peptide Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 (1) proved to be a very potent agonist (IC50 = 0.24 nM) of the MC4R receptor. This can be seen as the result of the cyclic constraints that position the DPhe-Arg-Trp fragment in a favorable geometry for interacting with the MC4 receptor.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Molecular Geometry of the Cyclic Peptide¶
To explore the bioactive conformation of the cyclic peptide 1, it was studied in an aqueous buffer and its structure was determined using 1H NMR spectroscopy. 35 low energy structures were calculated, based on 82 distance restraints between hydrogen atoms. Only eight of the lowest energy structures were used for the design of new agonists.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Design of Molecules with a Cyclohexane Core¶
Modeling analyses showed that the cyclohexyl scaffold was a good template for the design of non-peptide mimics. A cyclohexane molecule containing a tryptamine group at the 4-position, a butyl guanine at the 1-position and D-phenylalanine at the 1-position gives a good overlap with the Trp, Arg and DPhe moieties of peptide 1.
Acyl Groups to Keep the Compound Neutral¶
Since the backbone of peptide 1 has no charge, acyl groups were added to D-phenylalanine and to the β-nitrogen atom on the tryptamine to keep the compound neutral at those positions.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Cis and Trans Cyclohexane Isomers¶
The substituents on the cyclohexane can be either cis (2a) or trans (2b).
Cis Isomer¶
The cis isomer 2a can adopt one of two chair conformations, H(a) or H(b) being in the equatorial position.
Trans Isomer¶
The trans isomer 2b exists only as one chair conformation with H(a) and H(b) both being in the axial orientation.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Geometry of Cis Isomer with Tryptamine Equatorial¶
The following view shows the cis isomer having the tryptamine group in the equatorial orientation.
Geometry of Cis Isomer with Tryptamine Axial¶
The following view shows the cis isomer having the tryptamine group in the axial orientation.
Geometry of Trans Isomer: Substituents Equatorial¶
The following view shows the geometry of the trans isomer. In this molecule both substituents are in the equatorial orientation.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Cis Isomer Equatorial Aligned with Peptidic Agonist¶
The following view shows the overlap of the cis isomer of molecule 2 having the tryptamine in the equatorial orientation superimposed on the ensemble of conformations calculated with the NMR data. This conformation aligns well with the tripeptide DPhe-Arg-Trp region of the peptide agonist in 3D.
Cis Isomer Axial Aligned with Peptidic Agonist¶
The following view shows the overlap of the cis isomer of molecule 2 having the tryptamine in the axial orientation superimposed on the ensemble of conformations calculated with the NMR data. This conformation aligns well with the tripeptide DPhe-Arg-Trp region of the peptide agonist in 3D.
Trans Isomer Aligned with Peptidic Agonist¶
The following view shows the overlap of the trans isomer of molecule 2 having the tryptamine in the axial orientation superimposed on the ensemble of conformations calculated with the NMR data. This conformation aligns well with the tripeptide DPhe-Arg-Trp region of the peptide agonist in 3D.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Discovery of a Nanomolar Non-Peptidic Agonist¶
All three conformations of compound 2 overlap with the tripeptide region equally well. However, only the cis isomer was found active. The inactivity of the trans isomer cannot be explained by its overlap with the 3D structure of the cyclic peptide 1.
A Good Starting Point for Further Developments¶
In the binding assays this molecule was shown to be selective over the other sub-classes of receptors. This structure can be used as a starting point for the discovery and development of a more "drug-like" candidate compound having the proper physicochemical and pharmacokinetic properties.
articles
Design of a New Peptidomimetic Agonist for the Melanocortin. Receptors Based on the Solution Structure of the Peptide Ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH2 Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N and Baumgartner JW Bioorganic & Medicinal Chemistry Letters 13 2003
Case Study-4 : Renin Inhibitors¶
The Renin-Angiotensin System Cascade¶
The first event in the renin-angiotensin system (RAS) cascade, which leads to an increase in blood pressure, is the cleavage of angiotensinogen by the aspartyl protease renin. As a result, renin inhibitors are regarded as excellent agents for the treatment of hypertension. While many highly potent and selective inhibitors have been discovered, they never reached the market because of their poor oral bioavailability due to their peptide-based structures. Here we present the strategy developed to discover non-peptide inhibitors (which led to the commercialization of Aliskiren - for more details see the Aliskiren story in the chapter entitled "success stories in drug discovery").
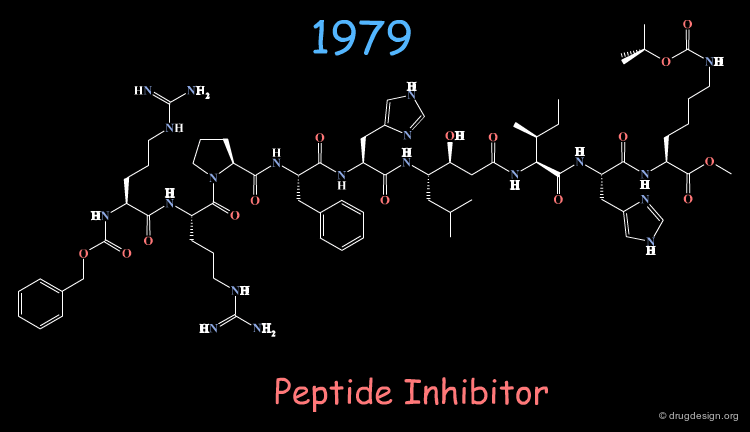
The First Generation of Renin Inhibitors¶
Knowledge of the sequence of the substrate fostered the discovery of potent peptidic renin inhibitors.
Example of Inhibitor¶
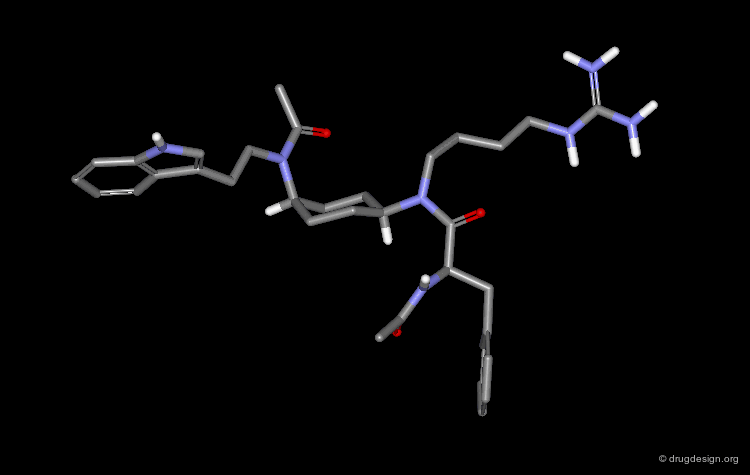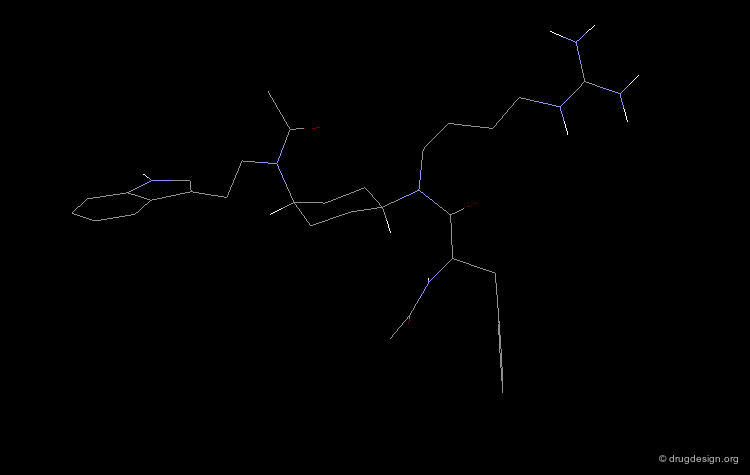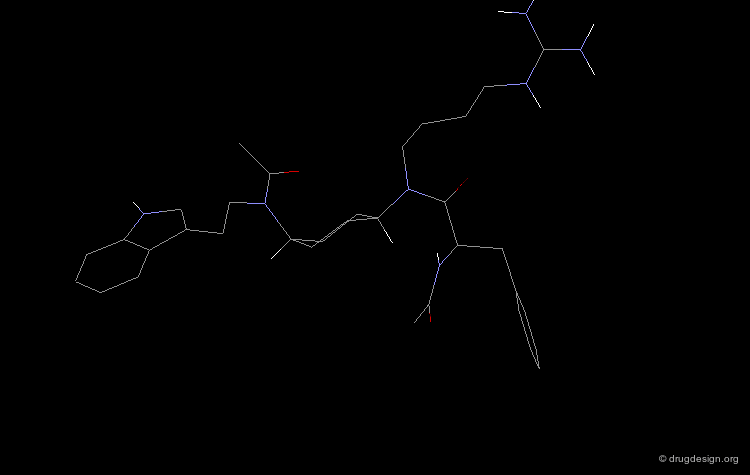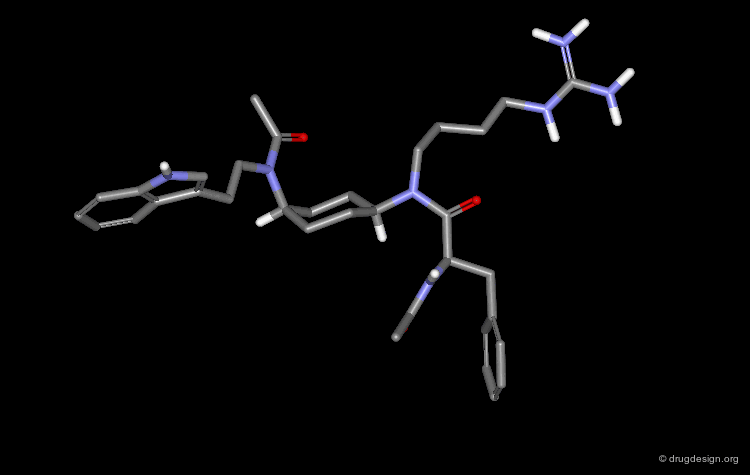
The molecule represented here is an example of inhibitor designed from the substrate sequence. However, such molecules with a molecular weight of about 2000 dalton could not be considered for further development.
The Second Generation of Renin Inhibitors¶
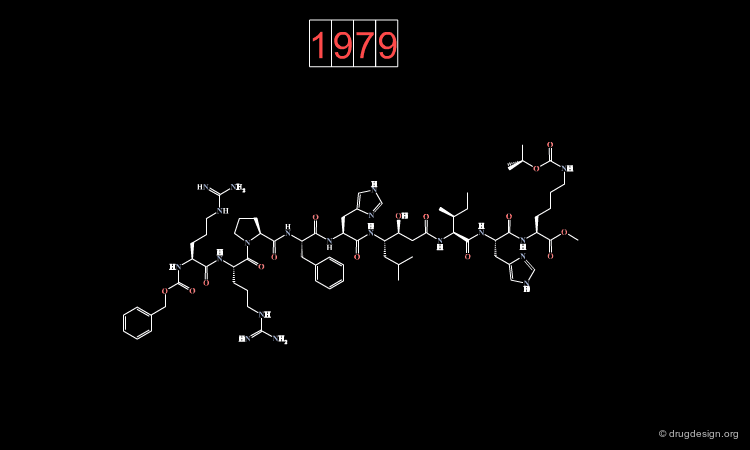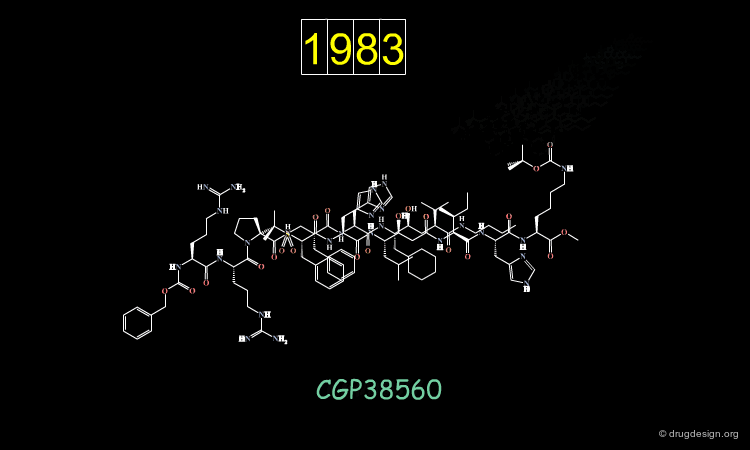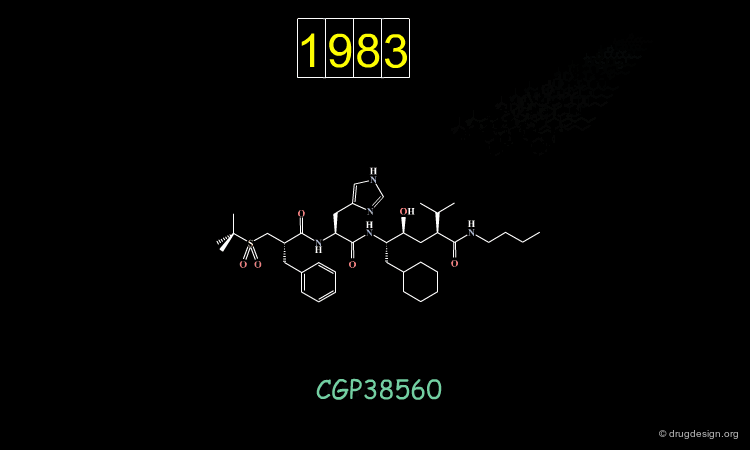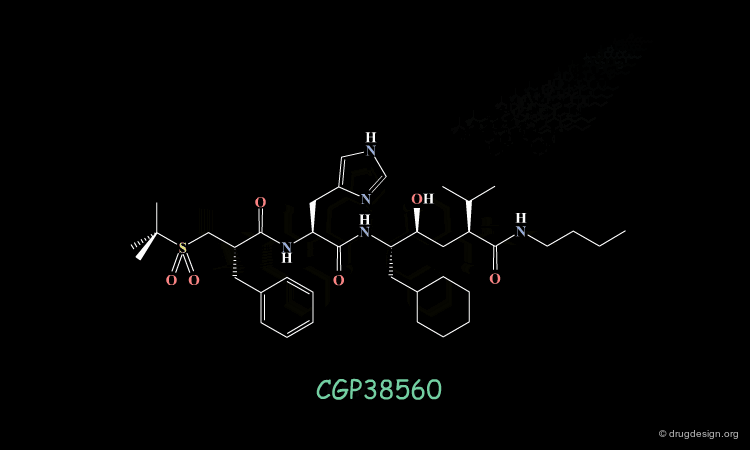
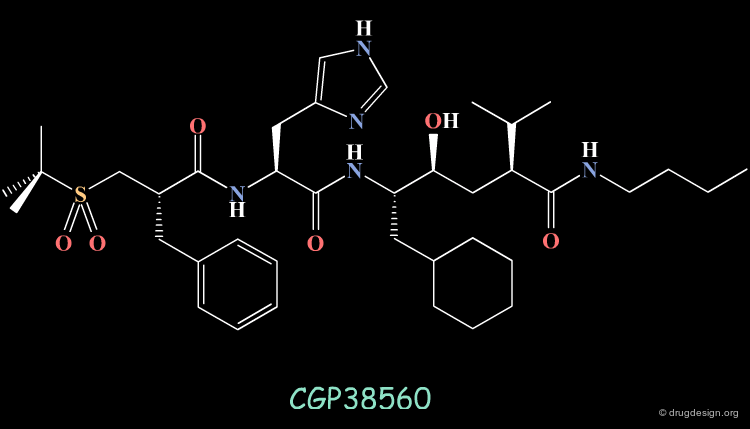
An important effort was then made in order to reduce the number of amino-acids. The molecule indicated here is an example of such achievements. CGP38560 is a very potent renin inhibitor (IC50 = 2 nM) with a relatively low molecular weight (730), however its clinical trials were very disappointing.
articles
Synthesis and Biological Activity of Some Transition-state Inhibitors of Human Renin Buhlmayer P, Caselli A, Fuhrer W, Goschke R, Rasetti V, Rueger H, Stanton JL, Criscione L and Wood JM J. Med. Chem. 31 1988
Synthesis and Biological Activity of Some Transition-state Inhibitors of Human Renin Buhlmayer P, Caselli A, Fuhrer W, Goschke R, Rasetti V, Rueger H, Stanton JL, Criscione L and Wood JM J. Med. Chem. 31 1988
Low Oral Absorption of CGP-38560¶
The compound showed low oral absorption (less than 1%) and rapid biliary excretion (half life of 7 minutes). The way to overcome the problems encountered was to develop a peptidomimicry strategy. The first step in this direction required the knowledge of the bioactive conformation of the reference peptide CGP38560. Based on a model of renin constructed by homology, docking simulations were conducted, starting each time with a different conformation of the inhibitor.
articles
Synthesis and Biological Activity of Some Transition-state Inhibitors of Human Renin Buhlmayer P, Caselli A, Fuhrer W, Goschke R, Rasetti V, Rueger H, Stanton JL, Criscione L and Wood JM J. Med. Chem. 31 1988
The Crystal Structures of Recombinant Glycosylated Human Renin Aone and in Complex with a Transition State Analog Inhibitor Rahuel J, Priestle JP and Grutter MG J. Struct. Biol. 107 1991
Computer Graphics Modelling of Human Renin. Specificity, Catalytic Activity and Intron-exon Junctions Sibanda BL, Blundell T, Hobart PM, Fogliano M, Bindra JS, Dominy BW and Chirgwin JM FEBS Lett. 174 1984
Bioactive Conformation of CGP-38560¶
The complex of lowest binding energy permitted the identification of the probable bioactive conformation of the peptide ligand. It was characterized by an extended conformation of the backbone. Most heteroatoms of the backbone were engaged in hydrogen bonding, in particular with the carbonyl of the Phe residue. Finally, the cyclohexyl and phenyl moieties of the side chains were close to each other occupying a large hydrophobic pocket in the enzyme.
articles
Structure-based drug design and the discovery of aliskiren (Tekturna): perseverance and creativity to overcome a R and D pipeline challenge Nissim Claude Cohen Chem Biol Drug Des 70(6) 2007 10.1111/j.1747-0285.2007.00599.x
book
N. Claude Cohen Trends in Medicinal Chemistry Elsevier Science Publisher 1989
Analysis of the Predicted Bioactive Conformation¶
Based on the predicted bioactive conformation of the peptide CGP38560 and additional analyses of the importance of the individual contributions of each group contributing to the binding, a strategy was considered for the design of non-peptidic compounds mimicking the reference peptide molecule.
articles
Structure-based drug design and the discovery of aliskiren (Tekturna): perseverance and creativity to overcome a R and D pipeline challenge Nissim Claude Cohen Chem Biol Drug Des 70(6) 2007 10.1111/j.1747-0285.2007.00599.x
Design and Syntheses of Novel 2,7-Dialkyl substituted5(S)-Amino-4(S)-Hydroxy-8-Phenyl-Octanecarboxamides as in vitro Potent Peptidomimetic Inhibitors of Humain Renin Goeschke R, Cohen NC, Wood JM and Maibaum J Bioorg. Med. Chem. Lett. 7 1997
Structure Based Drug Design: the Discovery of Novel Non-Peptide Orally Active Inhibitors of Human Renin Rahuel J, Rasetti V, Maibaum J, Rueger H, Goeschke R, Cohen NC, Stutz S, Cumin F, Fuhrer W, Wood JM and Gruetter M Chemistry & Biology 7 2000
Bioactive Hydroxyethylene Dipeptide Isosteres with Hydrophobic (P3-P1) - Moieties, A Novel Strategy Towards Small Non - Peptide Renin Inhibitors Rasetti V, Cohen NC, Rueger H, Goeschke R, Maibaum J, Cumin F, Fuhrer W and Wood JM Bioorg. Med. Chem. Lett. 6 1996
Strategy for the Design of Non-Peptidic Inhibitors¶
The following strategy was used: (1) removing the entire backbone from the cyclohexyl to the tert-butyl sulfone but keeping their anchorage using their two hydrogen bonds with the enzyme; (2) connecting directly the phenyl and cyclohexyl by a chemical link; (3) keeping unchanged the rest of the right part of the molecule (from the hydroxyl to the terminal butylamide fragment); (4) discarding the histidine side chain.
Successful Design of a Non-Peptidic Inhibitor¶
The following view shows an example of the successful design of a non-peptidic inhibitor mimicking the reference peptide inhibitor CGP38560. This series paved the way to a third generation of renin inhibitors.
articles
Structure Based Drug Design: the Discovery of Novel Non-Peptide Orally Active Inhibitors of Human Renin Rahuel J, Rasetti V, Maibaum J, Rueger H, Goeschke R, Cohen NC, Stutz S, Cumin F, Fuhrer W, Wood JM and Gruetter M Chemistry & Biology 7 2000
Structure-based drug design and the discovery of aliskiren (Tekturna): perseverance and creativity to overcome a R and D pipeline challenge Nissim Claude Cohen Chem Biol Drug Des 70(6) 2007 10.1111/j.1747-0285.2007.00599.x
Optimization of the Tetrahydroquinoline Inhibitor¶
The prototype non-peptidic molecule presented in the previous slide is active at the nanomolar range. The compound has a bicyclic tetrahydroquinoline moiety carrying an ester substituent. Notice the progression of the biological activities all along the construction of the entire substance: the molecule represented in white is active at the micromolar range; the introduction of the second ring (red) raises the biological potency by an order of magnitude; and finally the molecule with the side chain (yellow) is a potent inhibitor active at the nanomolar range.
articles
Structure-based drug design and the discovery of aliskiren (Tekturna): perseverance and creativity to overcome a R and D pipeline challenge Nissim Claude Cohen Chem Biol Drug Des 70(6) 2007 10.1111/j.1747-0285.2007.00599.x
A Third Generation of Renin Inhibitors¶
Using the same strategy and mimicking the reference peptide inhibitor (top), four chemically unrelated series of very potent non-peptidic renin inhibitors were discovered: the tetrahydroquinoline series (red), the indole (blue), the phenoxy (violet) and the salicylamide series (green). An analog of the phenoxy lead (Aliskiren) has been successfully developed and commercialized for the treatment of high blood pressure.
Alignment of the Non-Peptide Inhibitors in 3D¶
The tetrahydroquinoline series, the indole, the phenoxy, and the salicylamide series are aligned in this view. It illustrates the good molecular mimicry features between the different non-peptidic renin inhibitors.
Case Study-5 : Inhibitors of HLE¶
Inhibition of Human Leukocyte Elastase¶
Human Leukocyte Elastase (HLE) is a serine protease that is released by polymorphonuclear leukocytes in response to inflammatory stimuli. It has been postulated that inhibitors of elastase could prove therapeutically useful against diseases such as adult respiratory distress syndrome, cystic fibrosis and emphysema. A peptide trifluoromethyl ketone (TFMK) molecule was discovered which inhibited HLE.
Problem of Peptide-Based ICI-200,880¶
The systematic chemical modification of the trifluorometyl ketone peptide (TFMK) has lead to molecule ICI-200,880 who is a potent inhibitor of HLE. In clinical trials, the molecule showed long-lasting protection against HLE-induced lung damage, but unfortunately it has shown very poor levels of oral activity. Efforts have been made to decrease the peptide character of ICI-200,880.
articles
Biologic Characterization of ICI 200,880 and ICI 200,355, Novel Inhibitors of Human Neutrophil Elastase Williams JC, Falcone RC, Knee C, Stein RL, Strimpler AM, Reaves B, Giles RE and Krell RD Am. Rev. Respir. Dis. 144 1991
TFMK as a Reference¶
TFMK was used as a starting point for the development of the peptidomimetics approach with the intention of adding the additional useful elements of ICI-200,880 at a later stage. At the time of this project only the X-ray of the porcine pancreatic elastase (PPE) was available. PPE and HLE share 40% sequence similarity.
Analysis of the Binding of TFMK (1/4)¶
The crystallographic studies of the TFMK inhibitor complexed with PPE revealed the following key inhibitor-enzyme interactions: Ser-195 is covalently attached to the TFMK carbonyl. The resulting oxyanion is stabilized by hydrogen bond to the backbone amide N-H of both Ser-195 and Gly-193.
articles
X-ray Diffraction Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Trifluoromethylketone Takahashi LH, Radhakrishnan R, Rosenfield RE Jr, Meyer EF Jr, Trainor DA and Stein M J. Mol. Biol. 201 1988
Analysis of the Binding of TFMK (2/4)¶
The isopropyl group of the Valine occupies the S1 specificity pocket.
articles
X-ray Diffraction Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Trifluoromethylketone Takahashi LH, Radhakrishnan R, Rosenfield RE Jr, Meyer EF Jr, Trainor DA and Stein M J. Mol. Biol. 201 1988
Analysis of the Binding of TFMK (3/4)¶
The Proline occupies the S2 pocket.
articles
X-ray Diffraction Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Trifluoromethylketone Takahashi LH, Radhakrishnan R, Rosenfield RE Jr, Meyer EF Jr, Trainor DA and Stein M J. Mol. Biol. 201 1988
Analysis of the Binding of TFMK (4/4)¶
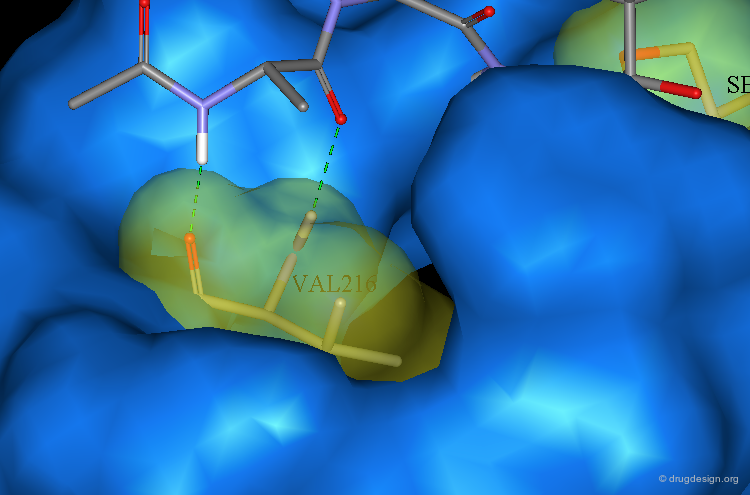
A pair of β-sheet hydrogen bonds are formed between the Alanine and Val-216.
articles
X-ray Diffraction Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Trifluoromethylketone Takahashi LH, Radhakrishnan R, Rosenfield RE Jr, Meyer EF Jr, Trainor DA and Stein M J. Mol. Biol. 201 1988
Summary of the Analyses¶
Ser-195 is covalently attached to the TFMK carbonyl. The resulting oxyanion is stabilized by hydrogen bonds to the backbone amide N-H of both Ser-195 and Gly-193. The isopropyl group of Valine occupies the S1 specificity pocket. The Proline occupies the S2 pocket. A pair of β-sheet hydrogen bonds are formed between the Alanine and Val-216.
articles
X-ray Diffraction Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Trifluoromethylketone Takahashi LH, Radhakrishnan R, Rosenfield RE Jr, Meyer EF Jr, Trainor DA and Stein M J. Mol. Biol. 201 1988
Design of a New Pyridone Framework¶
Analysis of the conformation of P2 and P3 residues of the bound inhibitor in this model suggest that it would be possible to build a hydrocarbon bridge from it to the adjacent proline without disrupting the critical hydrogen bonding interactions with Val-216.
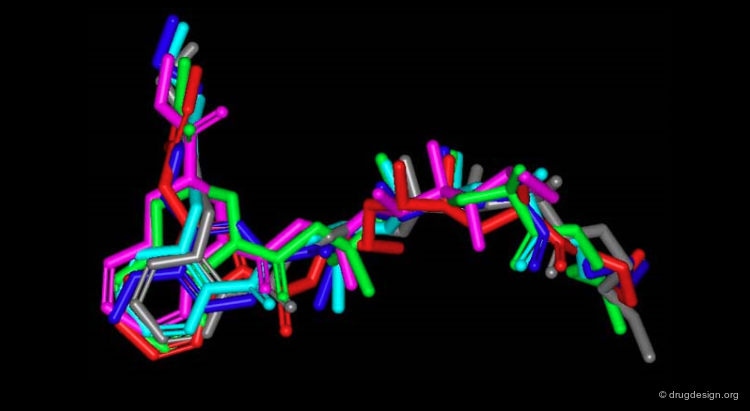
3D Superimposition with TFMK¶
The superimposition of the new molecule with the reference peptide TFMK is displayed below. It shows how the designed compound superimposes well with the peptidic backbone of TFMK.
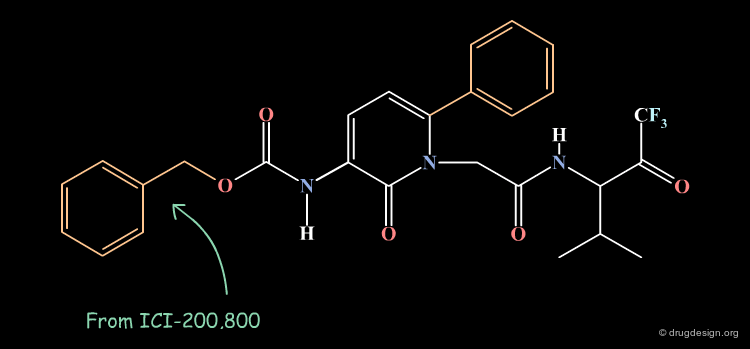
Synthesis of Pyridone Molecule¶
Analysis of the new compound has shown that the removal of the proline ring retained the conformation of the molecule. Pyridone was synthesized and showed a Ki similar to the one observed on TFMK.
3D Geometry Maintained after Removal of Proline¶
From the superimposition of the originally designed bicyclic molecule and the pyridone, one can observe that the removal of the proline did not affect the conformation of the molecule. The first step of the peptidomimicry design has been achieved. It was possible to replace the peptidic backbone by a regular organic scaffold. The next step to be considered is the optimization of the pyridone molecules.
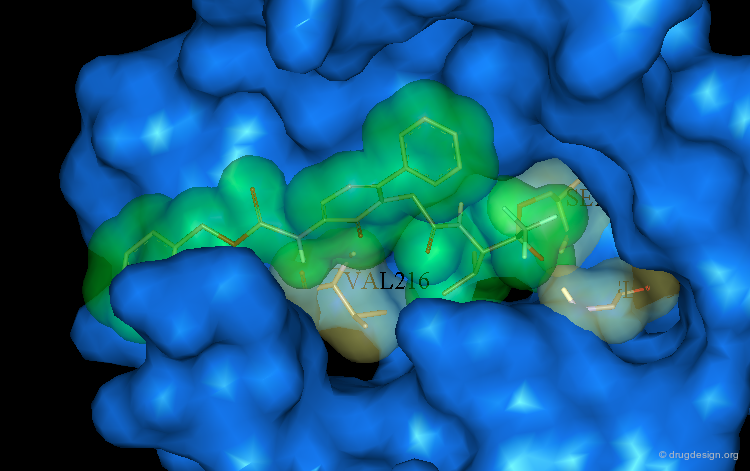
Analysis of the Pyridone Bound to PPE¶
Analysis of the binding of the pyridone molecule into the catalytic site of PPE suggests that the addition of a phenyl ring as a substituent of the pyridone cycle may contribute to increase the binding to the enzyme.
Optimization of the Pyridone Series¶
Moreover the interesting structural elements already identified in ICI-200,880 were incorporated in the pyridone series. The resulting compound has proved to be a very potent inhibitor of HLE with a Ki of 4 nM.
Browser of HLE Inhibitors¶
ADDITIONAL CASE STUDIES¶
Additional Case Studies¶
Copyright © 2024 drugdesign.org