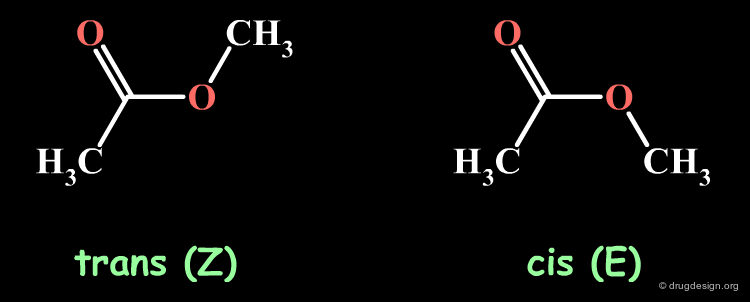Selected Examples in 3D Analysis¶
Info
The conformational features of small-molecule systems such as linear, cyclic, conjugated, aromatic molecules will be presented and discussed.
Number of Pages: 49 (±1 hours read)
Last Modified: January 2004
Prerequisites: Molecular Geometry, Molecular Energies, Conformational Analysis
Conformational Analysis¶
Ethane¶
The potential energy for the rotation of the carbon-carbon bond in ethane is presented here as a function of the torsion angle.
articles
Conformations of Substituted Ethanes Kingsbury CA J. Chem. Education 56(7) 1979
Internal Barrier in Ethane Lide DR Jr J. Chem. Phys. 29 1958
The Barrier to Internal Rotation in Ethane Lowe JP Science 179 1973
Origin of the Gauche Effect in Substituted Ethanes and Ethenes Wiberg KB, Murcko MA, Laidig KE and MacDougall PJ J. Phys. Chem. 94 1990
n-Butane¶
The potential energy for the rotation of the central carbon-carbon bond in n-butane is presented here as a function of the torsion angle.
articles
The syn Rotation Barrier in Butane Allinger NL, Grev RS, Yates BF and Schaefer HF III J. Am. Chem. Soc. 112 1990
Rotational Barrier. 2. Energies of Alkane Rotamers. An Examination of Gauche Interactions Wiberg KB and Murcko MA J. Am. Chem. Soc. 110 1988
1-Butene¶
The potential energy for the rotation of the central carbon-carbon bond in 1-butene is presented here as a function of the torsion angle.
book
Eliel EL and Wilen SH Stereochemistry of Organic Compounds John Wiley & Sons Inc 1994
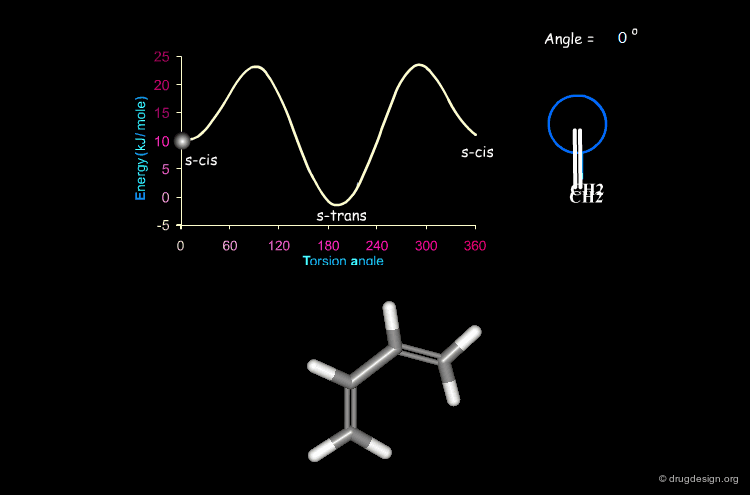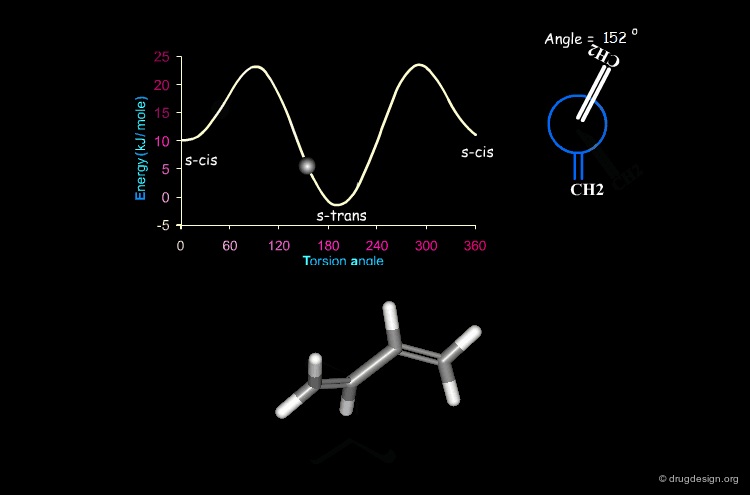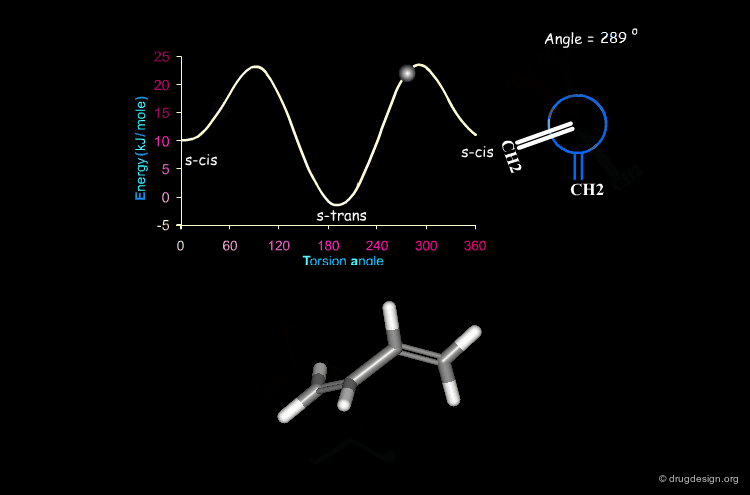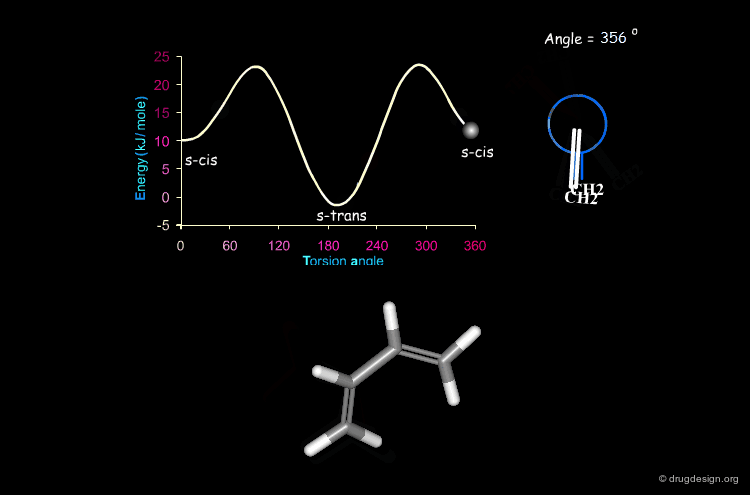
Butadiene¶
The potential energy for the rotation of the central carbon-carbon bond in butadiene is presented here as a function of the torsion angle.
book
Eliel EL and Wilen SH Stereochemistry of Organic Compounds John Wiley & Sons Inc 1994
Amide¶
The potential energy for the rotation of the central carbon-nitrogen amide bond is presented here as a function of the torsion angle.
articles
Conformational Analysis of Some Alcohols and Amines: A Comparison of Molecular Orbital Theory, Rotational and Vibrational Spectroscopy Truax DR and Wieser H Chem. Soc. Rev. 6 1977
Cyclohexane¶
The potential energy profile of the cyclohexane ring inversion is presented here. Click on the graph to visualize the corresponding conformer.
articles
Chair-Chair Interconversion of Six Membered Rings Anderson JE Top. Curr. Chem. 45 1974
Ring Inversion in Cyclohexane Anet FAL, Ahmad M and Hall LD Proc. Chem. Soc.
1964
Molecular Geometry. VII. Modes of Interconversion in the Medium Rings Hendrickson JB J. Am. Chem. Soc. 99 1967
The Rate of Chair-Chair Interconversion of Cyclohexane Jensen FR, Noyce DS, Sederholm CH and Berlin AJ J. Am. Chem. Soc. 84 1962
Spectroscopic Detection of the Twist-Boat Conformation of Cyclohexane. A Direct Measurement of the Free Energy Difference Between the Chair and the Twist-Boat Squillacote M, Sheridan RS, Chapman OL and Anet FAL J. Am. Chem. Soc. 97(11) 1975
Conjugated Systems¶
Butadiene¶
When two double bonds are separated by a single bond (conjugated system), they tend to be coplanar, a geometry that maximizes the overlap of the π orbitals of the system. The coplanarity is achieved in the s-cis and the s-trans conformations of the conjugated fragment. The s-trans conformer is preferred by 11.5 kJ/mol.
articles
A New Indo-type Procedure for Conjugated Non-rigid Molecules. 1. Ground-State Conformations and Barriers to Internal Rotation Momicchioli F, Baraldi I and Bruni MC Chem. Phys. 70 1982
Conformational Analysis. 120. Small Polyenes Tai JC and Allinger NL J. Am. Chem. Soc. 98(25) 1976
Pentenone¶
However, in Z-pent-3-ene-2-one the s-cis conformer is preferred (by about 10 kJ/mol) because of the steric repulsion occurring between the two methyl groups when the molecule is in the s-trans conformation.
Dipyrrole¶
When two aromatic rings are connected by a single bond, the molecule adopts a planar conformation as long as there are no severe steric repulsions. Dipyrrole is a planar molecule.
Biphenyl¶
Biphenyl cannot be planar, due to steric repulsions between the ortho hydrogens on the aromatic rings. A rotation of about 35° occurs around the single bond where a compromise is found between the steric and the optimal conjugative effects. In this conformation the molecule is partially deconjugated.
articles
A New Indo-type Procedure for Conjugated Non-rigid Molecules. 1. Ground-State Conformations and Barriers to Internal Rotation Momicchioli F, Baraldi I and Bruni MC Chem. Phys. 70 1982
The Determination of Absolute Configuration of Planar and Axially Dissymmetric Molecules Krow G Topics in Stereochemistry 5 1970
Atropisomerism of Biphenyls¶
Most tetra ortho substituted biphenyls are optically resolvable. This is due to the restricted rotation about the single bond. This phenomena is called "atropisomerism", the rotational barrier is high enough to permit isolation of the two isomers. An example of enantiomeric chiral biphenyls is visualized here.
articles
The Determination of Absolute Configuration of Planar and Axially Dissymmetric Molecules Krow G Topics in Stereochemistry 5 1970
Binaphthyl¶
The steric repulsions in binaphthyl are so severe that the molecule adopts a conformation with a torsion angle of 66°. There is no conjugation left and the aromatic rings are almost orthogonal.
articles
Molecular Mechanics Calculation in Organic Chemistry: Examples of the Usefulness of this Simple Non-quantum Mechanical Model Osawa E and Musso H Angew. Chem. Int. Ed. Engl. 22 1983
Aromatic Systems¶
Planarity of Polyaromatic Systems¶
A polyaromatic system tends to be planar for an optimal overlap of the π orbitals of the rings.
articles
The Synthesis of Corannulene Barth WE and Lawton RG J. Am. Chem. Soc. 93(7) 1971
The Crystal Structure of [18]annulene. I. X-ray Study Bregman J, Hirshfeld FL, Rabinovich D and Schmidt GMJ Acta Cryst. 19 1965
The Crystal Structure of [18]annulene. II. Results Hirshfeld FL and Rabinovich D Acta Cryst. 19 1965
Synthesis and Molecular Structure of [7]Circulene Yamamoto K, Harada T, Okamoto Y, Chikamatsu H, Nakazaki M, Kai Y, Nakao T, Tanaka M, Harada S and Kasai N J. Am. Chem. Soc. 110(11) 1988
Distorted Naphthalene¶
When bulky substituents are introduced in the α positions, they alter the planarity (therefore the conjugation) of the molecule. The following view illustrates the distorded geometry adopted by 1,8-di-t-butylnaphthalene, the angle between the two substituents is about 68°.
articles
Dramatic Steric Distortion and Electronic Demands in 1,3,5-Tris(dialkylamino)-2,4,6-trinitrobenzene: Study of a Severely Warped Benzene Chance JM, Kahr B, Buda AB and Siegel JS J. Am. Chem. Soc. 111 1989
Annelated Polyaromatic Benzenes¶
By fusing a benzene ring on the α-β position of naphthalene one gets the phenanthrene molecule. This compound has an entirely flat geometry.
articles
Specification of Molecular Chirality Cahn RS, Ingold C, Prelog V Angew. Chem. Int. Ed. Engl. 5 1966
The Determination of Absolute Configuration of Planar and Axially Dissymmetric Molecules Krow G Topics in Stereochemistry 5 1970
Synthesis and Structure of Longitudinally Twisted Polycyclic Aromatic Hydrocarbons Pascal RA Jr, McMillan WD, Van Engen D and Eason RG J. Am. Chem. Soc. 109 1987
Synthesis, Structure and Basicity of 1,16-Diaza[6]helicene Staab HA, Diehm M and Krieger C Tetrahedron Lett. 35(45) 1994
Fusing Another Ring¶
By fusing another ring one gets a fused ring system with steric repulsions. These repulsions are relieved by a small alteration of the planarity of the polyaromatic system.
Fusing Again Another Ring¶
The molecule obtained with the fusion with another ring has more severe steric repulsions, which increases the deviation from the planarity of this five ringed fused system.
Continue to Fuse Additional Rings¶
The effect is amplified by fusing additional rings. Such polyaromatic annelated systems adopt a helical geometry in which the steric repulsions are reduced to the minimum. This structure is aromatic, non-planar and chiral; the (-) helix is visualized here.
Cyclic Systems¶
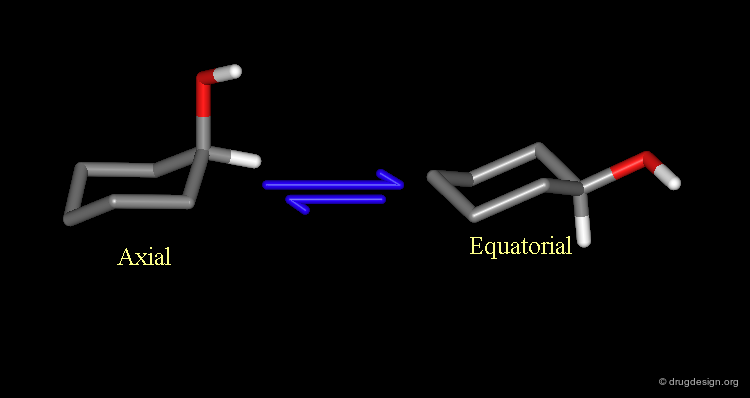
Why Substituents Prefer to be Equatorial?¶
Due to the existence of two possible chair conformations of the ring, the substituent of mono-substituted cyclohexanes can be either in the equatorial or in the axial orientation. However because of the 1,3 diaxial repulsions in the axial conformer the equatorial conformer is preferred.
Mono-Substituted Cyclohexanes¶
The equatorial conformer is preferred because of the strong van der Waals repulsions that exist in the axial conformer. The greater the size of the substituent, the higher the difference of energy between the two forms.
articles
Molecular Mechanics: The MM3 Force Field for Hydrocarbons. 1 Allinger NL, Yuh YH and Lii JH J. Am. Chem. Soc. 111(23) 1989
Neighboring Carbon and Hydrogen. XIX. t-Butylcyclohexyl Derivatives. Quantitative Conformational Analysis Winstein S and Holness NJ J. Am. Chem. Soc. 77 1955
t-Bu¶
The equatorial conformer is preferred to the axial one, with a difference of energy of 24.7 kJ/mol.
Phenyl¶
The equatorial conformer is preferred to the axial one, with a difference of energy of 12.5 kJ/mol.
Methyl¶
The equatorial conformer is preferred to the axial one, with a difference of energy of 7.1 kJ/mol.
Hydroxy¶
The equatorial conformer is preferred to the axial one, with a difference of energy of 2.1 kJ/mol.
Example of Preferred Axial Conformer¶
Axial substituents introduce strain energies because of the 1,3 diaxial repulsions. However, if for some reason there are no interactions of this type, the axial conformer becomes more populated. This is the case of the molecule represented here. The preferred conformer has the tert-butyl group in the axial orientation and the difference of energy between the equatorial and the axial conformers is 6 kJ/mol.
articles
Axial Monoalkyl Cyclohexanes Biali SE J. Org. Chem. 57(11) 1992
Conformational Analysis. XVI. 1-3Dioxanes Eliel EL and Knoeber MC Sr J. Am. Chem. Soc. 90(13) 1968
Conformational Aanlysis. XVIII. 1,3-Dithianes. Conformational Preference of Alkyl Substituents and the Chair-Boat Energy Difference Eliel EL and Hutchins RO J. Am. Chem. Soc. 91(10) 1969
Di-Methyl-1,2-Cyclohexane¶
In di-methyl-1,2-cyclohexane the methyl groups can be either "cis" or "trans". In the "trans" isomer the preferred conformation is the one where the two substituents are in the equatorial orientation. In the "cis" isomer the two conformations are equally populated having one methyl group in the axial and the other in the equatorial orientation.
Trans¶
The difference of energy is 10.8 kJ/mol in favor of the equatorial conformer.
Cis¶
The two conformations are equally populated.
Di-Methyl-1,3-Cyclohexane¶
In di-methyl-1,3-cyclohexane the methyl groups can be either "cis" or "trans". In the "cis" isomer the preferred conformation is the one where the two substituents are in the equatorial orientation. In the "trans" isomer the two conformations are equally populated having one methyl group in the axial and the other in the equatorial orientation.
articles
A Novel Attractive 1-3 Interaction in Trans-1,4-dichlorocyclohexane Abraham RJ and Rossetti ZL Tetrahedron Lett. (49) 1972
Trans¶
The two conformations are equally populated.
Cis¶
The difference of energy is 23.0 kJ/mol in favor of the equatorial conformer.
Di-Methyl-1,4-Cyclohexane¶
In di-methyl-1,4-cyclohexane the methyl groups can be either "cis" or "trans". In the "trans" isomer the preferred conformation is the one where the two substituents are in the equatorial orientation. In the "cis" isomer the two conformations are equally populated having one methyl group in the axial and the other in the equatorial orientation.
articles
A Novel Attractive 1-3 Interaction in Trans-1,4-dichlorocyclohexane Abraham RJ and Rossetti ZL Tetrahedron Lett. (49) 1972
Conformational Energies of Trans-1,4-difluoro- and Trans-1-chloro-4-fluorocyclohexane. The Role of Electrostatic Interaction Hammarstrom LG, Berg U and Liljefors T Tetrahedron Lett. 28(41) 1987
Trans¶
The difference of energy is 15.0 kJ/mol in favor of the equatorial conformer.
Cis¶
The two conformations are equally populated.
Trans 1,3-Di-t-Butyl-Cyclohexane¶
The two chair conformations of trans 1,3-di-t-butylcyclohexane cannot escape having one t-butyl in the axial orientation. In this case the boat conformation is preferred to the chair, with a difference of energy of about 1.5 kJ/mol.
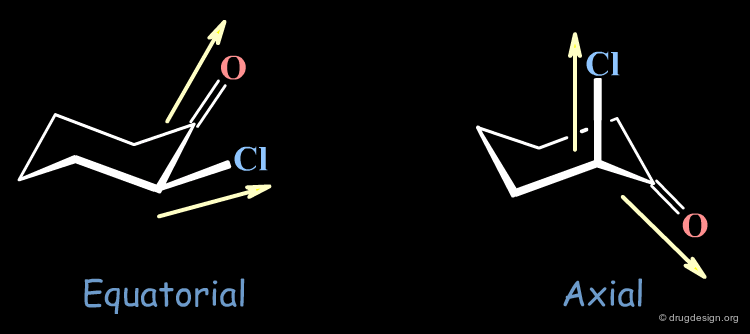
Chloro-2 Cyclohexanone¶
The difference of energy between the two conformers of chloro-2 cyclohexanone is 2.7 kJ/mol in favor of the axial conformer. This can be understood by the repulsion between the two dipoles C=O and C-Cl that are nearly parallel in the equatorial form. In non-polar solvents such as heptane, the axial conformer is dominant; however in polar solvents such as methanol, the equatorial conformation becomes preferred due to solvent dispersal (or moderating) of the dipole interactions.
articles
Rotational Isomerism. Part XV. The Solvent Dependence of the Conformational Equilibria in Trans-1,2 and Trans-1,4-Dihalogencyclohexanes Abraham RJ and Rossetti ZL J. Chem. Soc. Perkin. Trans. II.
1973
Other Systems¶
Decalins¶
Cis and trans decalins are molecules containing two cyclohexane rings fused together. The two rings can be fused in a cis or in a trans manner depending on the orientations of the angular hydrogens. In the cis decalin the two hydrogens are cis, and in the trans decalin they are trans.
articles
The molecular Structure of Cis- and Trans-bicyclo[4.4.0]decane in the Gas Phase, Studied by Electron Diffraction and Molecular Mechanics Van Den Enden L, Geise HJ and Spelbos A J. Mol. Struct. 44 1978
Preference for a Conformation With Axial t-butyl Group: 8β-t-butyl-cis-Decahydroquinoline Vierhapper FW Tetrahedron Lett. 22(51) 1981
Cis-decalin¶
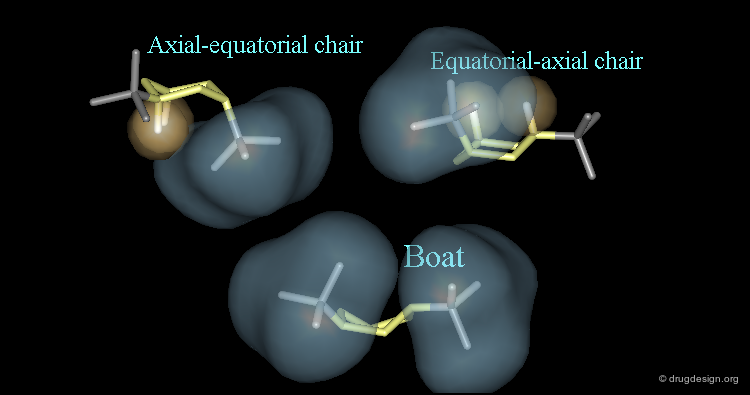
Cis-decalin has two non-identical interconverting chair-chair conformations. Each conformer is the mirror image of the other and the two forms are equally populated. Cis-decalin can also interconvert into chair-boat and boat-boat conformations, however they are of higher energies.
Methyl-Cis-decalin¶
The interconverting chair-chair conformations of cis-decalin changes an equatorial substituent into an axial orientation, and vice-versa. As a consequence substituted cis decalins prefer the substituent(s) in the equatorial orientation. For example, the preferred conformer of a methyl cis decalin is the one with the methyl group equatorial.
Trans-decalin¶
Trans-decalin has only one chair-chair conformation, it cannot invert to an alternative chair-chair conformation like cis decalin. Chair-boat and boat-boat conformations of trans decalin are theoretically possible but they have considerable higher energies.
Interactions of Aromatic Rings¶
Two benzene moieties have preferred interactions; some of them are illustrated here (note the offset stacking and the almost perpendicular orientations). Aromatic stacking is an important component in interactions in proteins and in DNA base pairs interactions.
articles
Aromatic-Aromatic Interaction: A Mechanism of Protein Structure Stabilization Burley SK and Petsko GA Science 229 1985
The Nature of π-π Interactions Hunter CA and Sanders JKM J. Am. Chem. Soc. 112 1990
Modeling Interaction with Benzene: Aryl-Aryl, Cation-π and Chaotrope-π Jorgensen WL, Severance DL and Duffy EM Computational Approaches in Supermolecular Chemistry 426 1994
Interactions Revealed by X-Ray Crystallography¶
A crystallographic structure of a protein reveals a great deal of information about the interactions between aromatic moieties. The following view illustrates the complex of a protein with a cofactor and an inhibitor; a stacking interaction is observed and highlighted here.
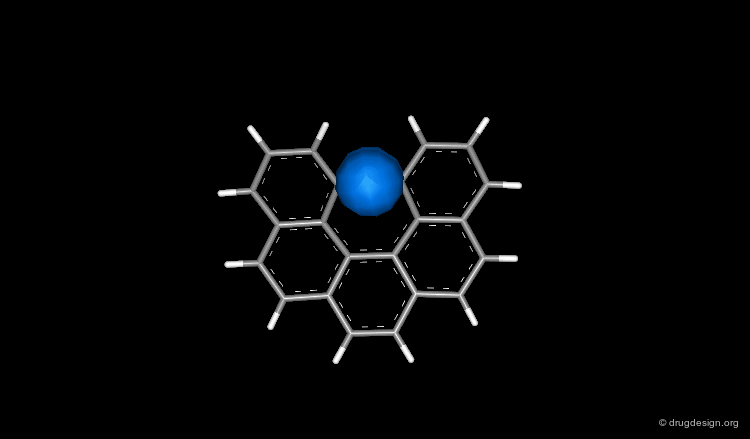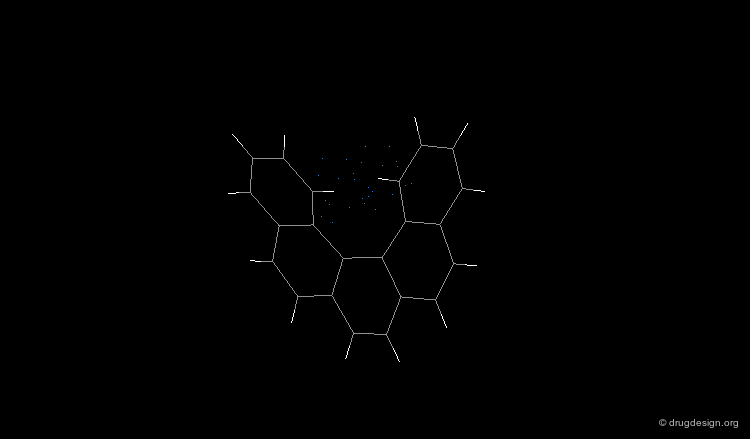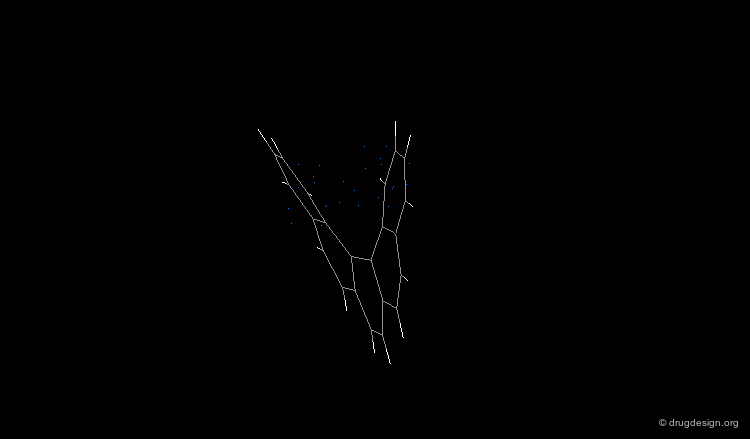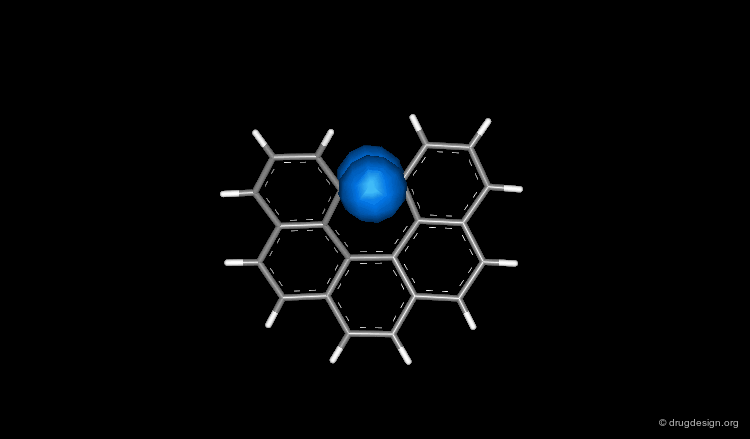
Two Major Types of Aromatic Interactions¶
Two types of aromatic interactions are highlighted here, the one involving a stacking and the other an orthogonal interaction.
Overview of Possible Interactions¶
Here are the interactions we mentioned in example 2; for clarity the rest of the structure was removed.
Geometry of Ester Groups¶
The ester moiety is planar. In principle it can exist in two geometries, the cis-(E) and the trans-(Z). However the E isomer is never observed in acyclic ester systems, the difference of energy between the E and the Z forms is so large that it is difficult to measure. Theoretical calculations indicate a difference of 25 kJ/mol in favor of the Z isomer.
articles
Microwave Spectrum, Barrier to Internal Rotation, and Structure of Methyl Formate Curl RF Jr J. Chem. Phys. 30(6) 1959
Molecular Structure and Conformation of Carboxylic Esters Jones GIL and Owen NL J. Mol. Struct. 18 1973
E and Z Conformations of Esters, Thiol Esters, and Amides Pawar DM, Khalil AA, Hooks DR, Collins K, Elliott T, Stafford J, Smoth L and Noe EA J. Am. Chem. Soc. 120 1998
152. Structural Characteristics of the Carboxylic Ester Group Schweizer WB and Dunitz JD Helv. Chim. Acta. 65 1982
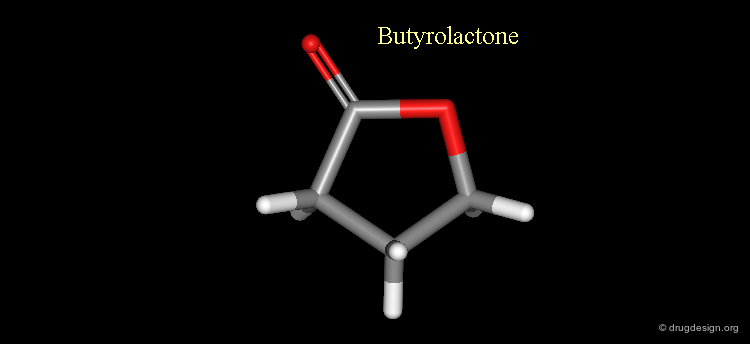
Cyclic Ester¶
An ester moiety is in the cis (E) conformation when it is part of a cyclic system (lactone structures). In this case the geometrical constraints of the ring force the ester group to adopt this geometry.
Geometry of Amide Groups¶
The amide moiety is planar. It can exist in the cis (E) or in the trans (Z) geometries, however there is a difference of energy of 10 kJ/mol in favor of the Z isomer. This means that the population of the E isomer is less than 3% at room temperature. Acyclic amide systems are therefore found in the Z geometry, in particular in protein structures.
articles
153. Structural Characteristics of the Carboxylic Amide Group Chakrabarti P and Dunitz JD Helv. Chim. Acta. 65 1982
Stereochemistry of N-Methylbenzanilide and Benzanilide Itai A, Toriumi Y, Tomioka N, Kagechika H, Azumaya I and Shudo K Tetrahedron Lett. 30(45) 1989
Molecular Structure of Acetamide as Studied by Gas Electron Diffraction Kitano M and Kuchitsu K Bull. Chem. Soc. Jpn. 46(10) 1973
Molecular Structure of N-methylformamide as Studied by Gas Electron Diffraction Kitano M and Kuchitsu K Bull. Chem. Soc. Jpn. 47(3) 1974
E and Z Conformations of Esters, Thiol Esters, and Amides Pawar DM, Khalil AA, Hooks DR, Collins K, Elliott T, Stafford J, Smoth L and Noe EA J. Am. Chem. Soc. 120 1998
Substituted Amide¶
If the nitrogen atom does not carry a hydrogen atom, then the two geometries E and Z become possible.
Cyclic Amide¶
An amide moiety is in the cis (E) conformation when it is part of a cyclic system (lactam structures). In this case the geometrical constraints of the ring, force the amide group to adopt this geometry.
Copyright © 2024 drugdesign.org